Valneva ’s VALN shares skyrocketed 39.8% on Oct 18 after the company announced positive top-line data from the pivotal phase III study Cov-Compare (VLA2001-301) evaluating its COVID-19 vaccine candidate, VLA2001.
The Cov-Compare study enrolled 4012 adult participants to compare VLA2001 with AstraZeneca ’s AZN COVID-19 vaccine AZD1222, which is already approved under emergency use across multiple nations.
Data from the study demonstrated the superiority of VLA2001 over the AstraZeneca vaccine. The study achieved both co-primary endpoints two weeks after the second vaccination. VLA2001 produced superior neutralizing antibody titer levels than AZD12222 and VLA2001 demonstrated the same effectiveness as AZD12222 in neutralizing antibody seroconversion rates above 95%.
Valneva’s shares have rallied 38.3% so far this year in comparison with the industry ’s 1% growth.
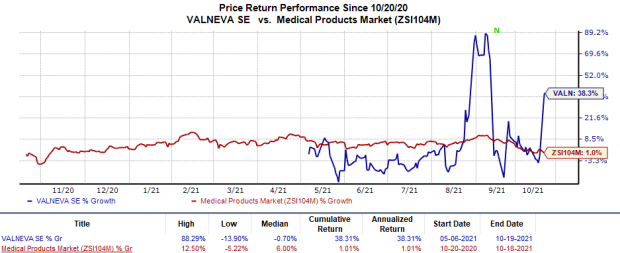 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
While the occurrence of COVID-19 cases was similar in participants administered either of the vaccines, patients provided with VLA2001 exhibited significantly better tolerability and safety profiles over patients who were given the AstraZeneca vaccine.
Per the company, VLA2001 is currently the only whole-virus, inactivated, adjuvanted vaccine candidate that is being evaluated in clinical studies for treating COVID-19 in Europe.
Data from the study also demonstrated that VLA2001 induced broad T-cell responses with antigen-specific IFN-gamma-producing T-cells against S, M and N proteins.
The company has already initiated a rolling submission for initial approval of VLA2001 with the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) earlier in August. MHRA has requested the company to provide a final assay validation to verify the data from the Cov-Compare study, which is also a pre-requisite of the clinical study report. Valneva is also preparing to initiate rolling submission for conditional approval in the European Union.
We note that the company is also preparing to evaluate VLA2001 in children aged between 5 and 12 years. It will also sponsor a booster study to evaluate VLA2001’s booster performance for people who are in need of a booster.
We remind investors that Valneva started trading on the NASDAQ stock exchange following its initial public offering on May 6, 2021.
Even though Valneva’s COVID-19 vaccine holds potential and receives potential approval, the vaccine faces stiff competition from those developed by AstraZeneca, Moderna MRNA , and Pfizer PFE /BioNTech, which are already in circulation across the world and have been already administered to billions of people. Pfizer’s COVID vaccine became the first to receive full approval in the United States in August 2021.
Zacks Rank
Valneva currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here .
Zacks’ Top Picks to Cash in on Artificial Intelligence
In 2021, this world-changing technology is projected to generate $327.5 billion in revenue. Now Shark Tank star and billionaire investor Mark Cuban says AI will create “the world’s first trillionaires.” Zacks’ urgent special report reveals 3 AI picks investors need to know about today.
See 3 Artificial Intelligence Stocks With Extreme Upside Potential>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report