Shares of
Valneva SE
VALN
were down 19.4% on Jun 13 after the company provided an update on its European inactivated whole-virus COVID-19 vaccine candidate VLA2001 on Jun 10.
VLA2001 is a whole virus, inactivated, adjuvanted vaccine candidate in clinical trials against COVID-19 in Europe.
Valneva has proposed a remediation plan after receiving the European Commission’s (“EC”) notice of intent to terminate the Advance Purchase Agreement (APA) for the vaccine last month.
The APA provides the EC with the right to terminate the agreement if, by Apr 30, 2022, VLA2001 did not receive marketing authorization from the European Medicines Agency (“EMA”). Valneva has 30 days from May 13, 2022, to obtain a marketing authorization or propose an acceptable remediation plan based on the terms of the APA.
Valneva now stated that the preliminary, unofficial volume indications received from the EC would not be sufficient to ensure the sustainability of the company’s COVID-19 vaccine program even though some member states have confirmed their interest in having an inactivated, adjuvanted whole-virus vaccine solution in their portfolio.
Hence, the EC is likely to terminate the agreement as Valneva will not be able to enter into an amendment to the APA that could allow for a reduced order.
The news disappointed investors. Valneva’s shares have plunged 69.4% in the year so far compared with the
industry
’s 26.2% fall.
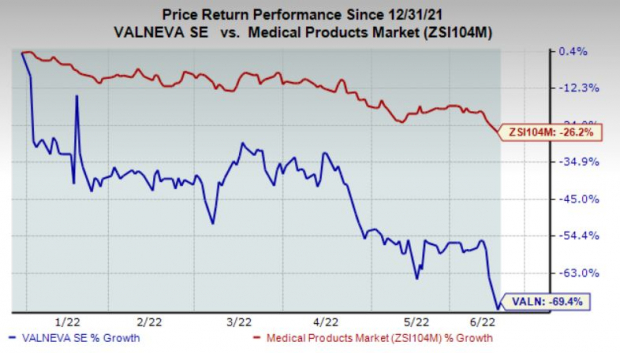
Image Source: Zacks Investment Research
Meanwhile, the EMA accepted the filing of the Marketing Authorization Application on May 19, 2022, and the Committee for Medicinal Products for Human Use is expected to take a final vote during the week of Jun 21, 2022.
Apart from VLA2001, Valneva is evaluating other vaccines in its pipeline for Lyme disease and chikungunya.
The company recently announced the successful completion of the lot-to-lot phase III study of its single-shot chikungunya vaccine candidate, VLA1553. The VLA1553-302 trial met its primary endpoint, demonstrating that three consecutively manufactured vaccine lots elicited equivalent immune responses measured by neutralizing antibody titer GMT ratios on Day 29 after vaccination.
VLA15, Valneva’s vaccine candidate for Lyme disease, is being developed in collaboration with
Pfizer
PFE
. Both Pfizer and Valneva plan to proceed with a phase III study of the vaccine in both pediatric and adult participants, which is expected to start in third-quarter 2022 based on the latest phase II immunogenicity and safety data.
We remind investors that Valneva collaborated with Pfizer in 2020 to develop and commercialize VLA15.
Valneva currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks are
Alkermes
ALKS
and
Geron Corporation
GERN
, both carrying a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
ALKS’ loss estimates for 2022 have narrowed to 3 cents from a loss of 14 cents in the past 60 days. Alkermes surpassed earnings in all the trailing four quarters, the average being 350.48%.
GERN’s loss estimates for 2022 have narrowed 6 cents in the past 60 days. Geron surpassed on earnings in three of the trailing four quarters and missed the mark in one, the average surprise being 1.07%.
How to Profit from the Hot Electric Vehicle Industry
Global electric car sales in 2021 more than doubled their 2020 numbers. And today, the electric vehicle (EV) technology and very nature of the business is changing quickly. The next push for future technologies is happening now and investors who get in early could see exceptional profits.
See Zacks’ Top Stocks to Profit from the EV Revolution >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








