Shares of
Novavax
NVAX
were up nearly 14% on Jun 23 after management announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (“CHMP”) recommended authorizing Nuvaxovid (NVX-CoV2373), the company’s protein-based COVID-19 vaccine, for use in adolescents aged between 12 and 17 years.
Per the CHMP, the benefits of the use of Nuvaxovid in adolescents outweigh its risks.
Nuvaxovid is
already authorized
in Europe for use in adults aged 18 years and older. This authorization was received by Novavax in December last year.
This positive opinion by the CHMP is based on data from the ongoing pediatric expansion of the phase III PREVENT study, which evaluated this protein-based vaccine in adolescents. The study achieved its primary effectiveness endpoint of NVX-CoV2373 generating neutralizing antibodies in adolescents, similar to the antibody responses in young adult participants (aged between 18 and 26 years), who were administered the vaccine in the phase III PREVENT study. In fact, the antibody responses were 1.5-fold higher in adolescents than young adults.
The results of this study are based on the data accrued between May 24 and Sep 27, 2021, the period during which the Delta variant was the dominating strain in the United States. NVX-CoV2373 demonstrated clinical efficacy of 80% against the Delta variant.
Shares of Novavax have plunged 63.8% so far this year compared with the
industry
’s 23% decline.
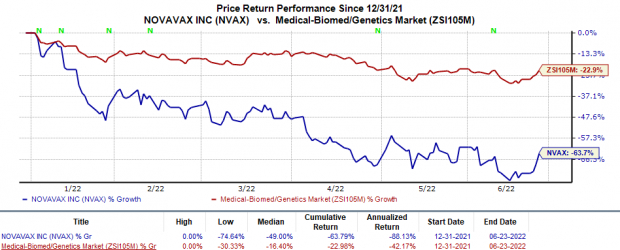
Image Source: Zacks Investment Research
If granted marketing authorization, Nuvaxovid will be the first protein-based vaccine for adolescents in Europe. This authorization will also help Novavax achieve its financial guidance for 2022. Management expects to record total revenues between $4 billion and $5 billion in the year.
In a separate press release, Novavax announced that it has received emergency use authorization for the use of Nuvaxovid in adults, in Taiwan. In another press release, NVAX announced that it has filed for label expansion in Canada for the use of its COVID vaccine in adolescents. In February, Nuvaxovid was granted authorization in Canada for use in individuals aged 18 years and older.
The Novavax vaccine is already authorized for use in more than 40 countries. NVAX is currently marketing two versions of its COVID-19 vaccine. NVX-CoV2373, which is marketed in partnership with the Serum Institute of India under the trade name Covovax, while the other is produced by NVAX itself and marketed under the trade name Nuvaxovid.
Nuvaxovid is yet to receive authorization for use in the United States. Earlier this month, the FDA’s Vaccines and Related Biological Products Advisory Committee
recommended granting
authorization to the Novavax vaccine for use in adults.
Once authorized by the FDA, NVX-CoV2373 will face stiff competition from the COVID-19 vaccines developed by
Moderna
MRNA
and
Pfizer
PFE
/
BioNTech
BNTX
. Novavax is already trailing a lot behind these vaccines. These vaccines dominate the U.S. market and are the only ones to have received full approval for use in adults in the country.
The two vaccines developed by Moderna and Pfizer/BioNTech are based on the mRNA technology, with high efficacy rates. In fact, the booster doses of the Moderna and Pfizer/BioNTech vaccines are also authorized for use in adults.
The vaccines developed by Moderna and Pfizer/BioNTech are currently the only ones authorized for use in individuals aged six months and above in the United States.
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +25.4% per year. So be sure to give these hand-picked 7 your immediate attention.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









