Biohaven Pharma
BHVN
announced the initiation of enrollment in a phase III study to assess the safety and efficacy of taldefgrobep alfa in patients with spinal muscle atrophy (SMA).
SMA is an inherited progressive neuromuscular disease in which the development and growth of muscle mass are compromised, leading to weakness and muscle atrophy, reduced motor function, impaired quality of life and death.
Taldefgrobep is an investigational, muscle-targeted recombinant protein, which, when combined with other approved treatments, has the potential to enhance muscle mass and strength in people living with SMA.
The phase III RESILIENT study, which expects to enroll 180 patients, will evaluate the safety and efficacy of taldefgrobep as an adjunctive therapy in patients already on a stable dose of
Biogen
’s
BIIB
Spinraza (nusinersen) or Roche’s Evrysdi (risdiplam) or with a history of treatment with onasemnogene abeparvovec-xioi in comparison to placebo.
Shares of Biohaven have returned 5.3% so far this year against the
industry
’s 20.5% decline.
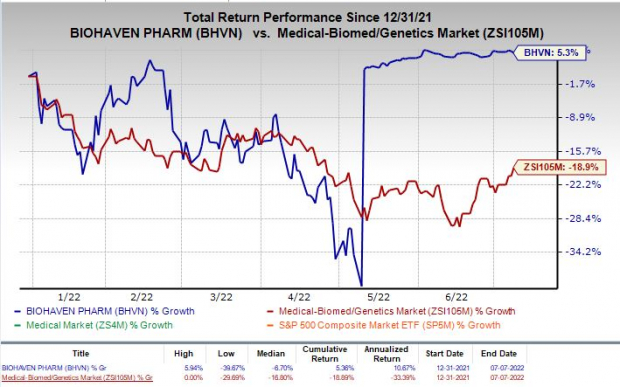
Image Source: Zacks Investment Research
Biogen’s SMA treatment, Spinraza (nusinersen) has strengthened its position in the neurological disease market, with the drug being the first treatment approved in the United States for SMA.
However, Biogen’s Spinraza has been witnessing a sales decline over the past two years. In the last reported quarter, sales of Spinraza declined 9% year over year to $472.5 million due to a decrease in demand as a result of increased competition
Roche received FDA approval for Evrysdi (risdiplam), its SMA drug, back in 2020. The drug has now been approved for babies below two months with SMA.
Biohaven gained development and commercialization rights to taldefgrobep alfa under a worldwide license agreement from
Bristol Myers Squibb
BMY
earlier this year
Per the agreement, Biohaven received global rights to taldefgrobep and Bristol Myers became eligible for regulatory approval milestone payments and tiered, sales-based royalties in the high teens.
Taldefgrobep is the third development asset from Bristol Myers, to be licensed out to Biohaven. Biohaven also in-licensed Nurtec and zavegepant, in 2016 from Bristol-Myers.
We request investors to note that in May, Biohaven and
Pfizer
PFE
signed an agreement wherein the latter will acquire the former for $148.50 per share or an aggregate equity value of $11.6 billion earlier this month.
Pfizer has already acquired the commercial rights to Biohaven’s sole marketed drug — Nurtec ODT — and calcitonin gene-related peptide (CGRP) pipeline candidates in ex-U.S. markets. These rights were acquired by PFE in November 2021, when it entered into a strategic collaboration agreement with BHVN.
The company will continue to operate under Biohaven’s name after the acquisition. PFE will also pay off BHVN’s third-party debt and redeem the latter’s outstanding preferred stock.
The acquisition deal, unanimously approved by the boards of directors of Pfizer and Biohaven, is subject to customary closing conditions, including approval from Biohaven’s shareholders and regulatory authorities. The transaction is expected to be completed by early 2023.
Zacks Rank
Biohaven currently carries a Zacks Rank #3 (Hold).
You can see
the complete list of today’s Zacks #1 Rank stocks here
.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2022?
From inception in 2012 through 2021, the
Zacks Top 10 Stocks
portfolios gained an impressive +1,001.2% versus the S&P 500’s +348.7%. Now our Director of Research has combed through 4,000 companies covered by the Zacks Rank and has handpicked the best 10 tickers to buy and hold. Don’t miss your chance to get in…because the sooner you do, the more upside you stand to grab.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









