Ultragenyx Pharmaceutical Inc.
RARE
, along with partner GeneTx Biotherapeutics LLC, announced an update from the phase I/II study evaluating GTX-102 for the treatment of Angelman syndrome, a rare neurogenetic disorder.
Angelman syndrome is caused by the loss of function of the maternally inherited Ubiquitin Protein Ligase E3A (UBE3A) gene.
The update included positive interim data from the above-mentioned open-label, dose-escalating phase I/II study that evaluated GTX-102 in pediatric patients having genetically confirmed diagnosis of full maternal UBE3A gene deletion.
Interim data from the study showed that treatment with GTX-102 led to a meaningful improvement in clinical disease and had an acceptable safety profile in nine patients from the United Kingdom and Canada arm and two patients from the United States arm of said study.
Per the company, these interim data support a protocol amendment to the above-mentioned phase I/II study, which was approved by the U.K. and Canadian health authorities in May 2022 to begin additional new cohorts of patients at higher monthly loading doses.
Along with the update, Ultragenyx also exercised its option to acquire GeneTx for an upfront payment of $75 million plus future milestone and royalty payments. The company has completed the acquisition.
The acquisition is likely to strengthen Ultragenyx’s broad portfolio of pipeline candidates that are being developed for treating various diseases.
Shares of Ultragenyx have lost 27.4% so far this year compared with the
industry
’s decline of 20.4%.
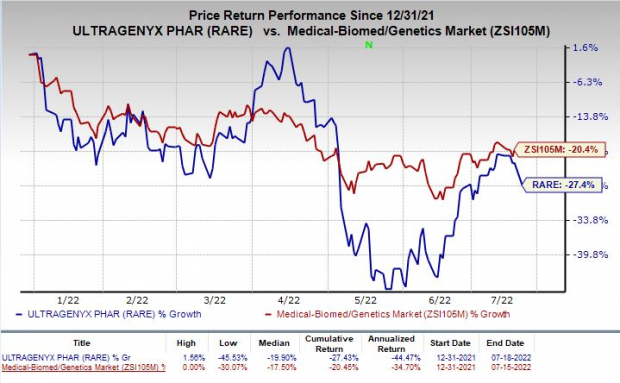
Image Source: Zacks Investment Research
In October 2020, Ultragenyx announced interim data from the phase I/II study on GTX-102 for treating Angelman syndrome. Back then, the company decided to cease enrollment and dosing in the study after all patients experienced a serious adverse event of lower extremity weakness related to local inflammation, following treatment with the highest doses of GTX-102.
In September 2021, the FDA removed the clinical hold from Ultragenyx and GeneTx’s phase I/II study evaluating GTX-102 for the treatment of Angelman syndrome.
In October 2021, Ultragenyx and GeneTx dosed the first patient in a phase I/II study in Canada, evaluating GTX-102 for the treatment of Angelman syndrome.
Zacks Rank & Stocks to Consider
Ultragenyx currently carries a Zacks Rank #4 (Sell).
Better-ranked stocks in the biotech sector are
Anika Therapeutics, Inc.
ANIK
,
Fate Therapeutics, Inc.
FATE
and
Beam Therapeutics Inc.
BEAM
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
The Zacks Consensus Estimate for Anika Therapeutics’ loss per share has narrowed 14.4% for 2022 and 9.1% for 2023 in the past 60 days.
Earnings of Anika Therapeutics have surpassed estimates in each of the trailing four quarters. ANIK delivered an earnings surprise of 200.45%, on average.
Fate Therapeutics’ loss per share estimates narrowed 0.3% for 2022 and 0.5% for 2023 in the past 60 days.
Earnings of Fate Therapeutics have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions. FATE delivered an earnings surprise of -0.72%, on average.
Beam Therapeutics’ loss per share estimates narrowed 0.7% for 2022 and 0.8% for 2023 in the past 60 days.
Earnings of Beam Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion. BEAM delivered an earnings surprise of 1.80%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








