Gilead’s
GILD
wholly owned subsidiary Kite announced a global strategic collaboration with a clinical-stage biotechnology company, Arcellx, Inc.
ACLX
, to co-develop and co-commercialize the latter’s lead late-stage product candidate, CART-ddBCMA.
CART-ddBCMA is an investigational cell therapy product comprising autologous T cells that have been genetically modified to target multiple myeloma. The candidate is currently in phase II.
Kite focuses on cell therapy to treat and potentially cure cancer.
Per the terms, Arcellx will receive an upfront cash payment of $225 million and a $100 million equity investment, as well as other potential contingent payments. Kite and Arcellx will share development, clinical trial, and commercialization costs for CART-ddBCMA and will jointly commercialize the product. The profits of the deal will be split equally. The transaction is expected to close in the first quarter of 2023.
Kite will commercialize the product and Arcellx will receive royalties on sales outside the US. Kite will be responsible for the development and commercialization costs of any product under the collaboration that is not co-commercialized. Kite will be responsible for manufacturing post completion of the technical transfer.
Gilead’s Cell Therapy franchise, comprising Yescarta and Tecartus, is gradually gaining traction.
Kite also announced findings from two new analyses of the landmark ZUMA-7 trial of Yescarta (axicabtagene ciloleucel), the largest and longest follow-up of a CAR T-cell therapy versus standard of care (SOC) in patients with relapsed or refractory large B-cell lymphoma (r/r LBCL). The data were presented orally at the 2022 American Society of Hematology (ASH) Annual Meeting & Exposition.
Results showed a consistent benefit of Yescarta versus SOC in the second-line r/r LBCL setting, including for those who required subsequent third-line treatment and even for specific patient subgroups.
Concurrently, Gilead also announced a clinical collaboration with
ImmunoGen
IMGN
. Both companies have collaborated to evaluate the safety and anti-leukemia activity of pivekimab sunirine (pivekimab) in combination with magrolimab, a potential, first-in-class, investigational CD47 inhibitor, in patients with relapsed or refractory (R/R) CD123-positive acute myeloid leukemia (AML).
ImmunoGen’s pivekimab sunirine is a CD123-targeting ADC in clinical development for hematological malignancies.
The collaboration will lead to a new cohort in ImmunoGen’s 802 study and will evaluate pivekimab in combination with magrolimab in up to 42 patients with R/R CD123-positive AML. It is expected to be initiated in 2023.
The primary endpoint for this cohort is the complete response (CR) rate. ImmunoGen’s 802 study is an open-label, multicenter, phase Ib/II study to determine the safety and tolerability of pivekimab and assess the anti-leukemia activity of the agent when administered in combination with Vidaza (azacitidine) and/or Venclexta (venetoclax) in patients with relapsed and frontline CD123-positive AML.
Gilead is making efforts to develop its oncology business to diversify its revenue base as competition in the HIV business is stiff.
Gilead is having a good run as its shares have gained 21.1% in the year so far against the
industry
’s decline of 19.1%.
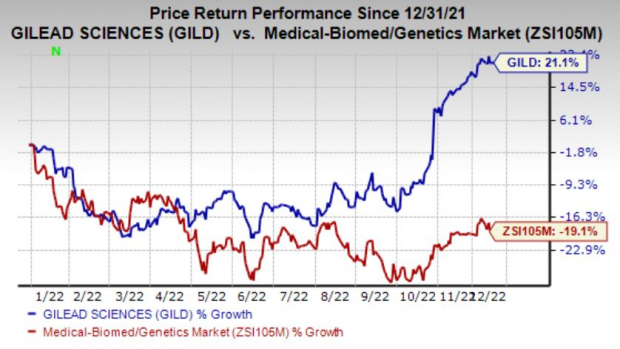
Image Source: Zacks Investment Research
The uptake of breast cancer drug Trodelvy has been strong and has boosted the top line.
The oncology space is lucrative and Gilead can capitalize on it, thereby creating a revenue growth driver in addition to the HIV franchise.
The company is also sitting on a huge cash balance. As of Sep 30, 2022, Gilead had $6.9 billion in cash, cash equivalents and marketable debt securities. Potential acquisitions to further expand its portfolio/pipeline will bode well for the stock.
However, competition is stiff in the HIV space from the likes of
GSK plc
GSK
and hence the approval of new treatments holds the key.
GSK’s HIV franchise recorded 7% growth in the third quarter. Growth was driven by new HIV products Dovato, Cabenuva, Rukobia, Juluca and Apretude and a favorable U.S. pricing mix.
Gilead currently carries a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Zacks Top 10 Stocks for 2023
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2023? From inception in 2012 through November, the Zacks Top 10 Stocks portfolio has tripled the market, gaining an impressive +884.5% versus the S&P 500’s +287.4%.
Now our Director of Research is combing through 4,000 companies covered by the Zacks Rank to handpick the best 10 tickers to buy and hold. Don’t miss your chance to get in on these stocks when they’re released on January 3.
Be First to New Top 10 Stocks >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








