Novavax
’s
NVAX
shares jumped 13% on Monday after the company announced that it had filed an Emergency Use Authorization (EUA) with the FDA for its protein-based COVID-19 vaccine, NVX-CoV2373.
This announcement comes exactly a month after Novavax
announced
that it completed the submission of the final data package — including the complete chemistry, manufacturing and controls (CMC) data module — with the FDA for NVX-CoV2373. The CMC submission is a prerequisite for the filing of EUA with the FDA. Per FDA guidance, the EUA application is required to be submitted at least one month after the filing of the final data package to the FDA.
Novavax’s filing with the FDA is based on data from two pivotal phase III studies on the vaccine — one (PREVENT-19) conducted in the United States and Mexico, and another in the United Kingdom. While the PREVENT-19 study achieved an overall vaccine efficacy of 90.4%, the data from the study conducted in the United Kingdom demonstrated that the vaccine achieved an overall efficacy of 89.7%.
We note that the FDA filing by Novavax comes after a series of delays. Earlier in May 2021, Novavax announced that its plans to file for the authorization for Novavax in the United States in second-quarter 2021 were delayed to the third quarter. The plan was further delayed in August 2021, when Novavax announced plans to delay this filing to fourth-quarter 2021. Finally, last December, Novavax announced that it would complete the filing by January-end.
Shares of Novavax have plunged 64.6% in the trailing 12 months in comparison with the
industry
’s 38% decline.
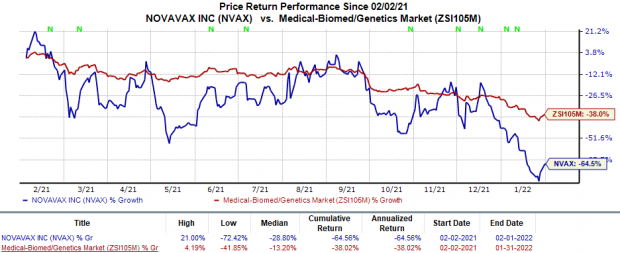
Image Source: Zacks Investment Research
We note that NVX-CoV2373 has already received approval for emergency use in multiple countries outside the United States. Novavax is currently marketing two versions of NVX-CoV2373 — one marketed in partnership with the Serum Institute of India under the trade name Covovax and another produced by NVAX that is marketed under the trade name Nuvaxovid.
While Covovax is already authorized for emergency use in India, Indonesia and the Philippines, Nuvaxovid has been granted approval for use in Australia, the European Union and South Korea.
Meanwhile, Novavax has submitted regulatory filings seeking approval for the NVX-CoV2373 in multiple markets like Canada, Japan, New Zealand, UAE and the United Kingdom. Novavax has already entered into advance purchase agreements with many of these nations for supplying doses of its COVID vaccine. A potential approval in any of these markets will give an impetus to the top line.
If granted EUA by the FDA, Novavax’s NVX-CoV2373 will be the fourth COVID vaccine authorized for use in the United States. The COVID vaccine market in the United States is dominated by three major pharma giants —
J&J
JNJ
,
Moderna
MRNA
and
Pfizer
PFE
/BioNTech — that have developed their own COVID-19 vaccines, posing a stiff competition to Novavx’s protein-based COVID vaccine.
We note that while the vaccines developed by Moderna and Pfizer/BioNTech are based on the mRNA technology and require a two-dose primary regimen, the vaccine developed by J&J is an anadenovirus-based vaccine only requiring a single-shot as primary regimen. The vaccines developed by these companies are not only approved for emergency use in the United States but also authorized for use in many countries.
In fact, the COVID vaccines developed by Pfizer and Moderna are currently the only ones that have received full approval in the United States. While Pfizer’s vaccine received full approval from the FDA for use in individuals aged 16 years of age and older last August, Moderna’s vaccine received full approval from the FDA in individuals aged 18 years and older this week.
Last December, the U.S. Centers for Disease Control and Prevention (“CDC”)
recommended
the use of mRNA-based COVID-19 vaccines or their booster doses over J&J’s COVID-19 vaccine. The CDC stated that J&J’s vaccine or its booster dose should be used only when mRNA-based vaccines are contraindicated for a person or are inaccessible.
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +25.3% per year. So be sure to give these hand-picked 7 your immediate attention.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









