San Diego-based
Mirati Therapeutics
MRTX
has been progressing well with the clinical development of its two pipeline candidates — adagrasib, a KRAS G12C inhibitor, and sitravatinib, a multi-kinase inhibitor.
While the company lacks a marketed drug in its portfolio, both adagrasib and sitravatinib have demonstrated the potential to be developed into drugs with a differentiated product profile.
Tumors characterized by KRAS mutations are extremely difficult to directly inhibit and are commonly associated with poor prognosis and resistance to therapy.
Mirati is currently evaluating adagrasib — both as monotherapy and combinations — in multiple cohorts of the phase I/II KRYSTAL-1 study across multiple solid tumors that carry KRAS G12C mutations. Based on data from a phase II registration-enabling cohort of the KRYSTAL-1 study, the company submitted a new drug application (NDA) to the FDA seeking approval for adagrasib, as monotherapy, to treat patients with at least second-line non-small cell lung cancer (NSCLC) with KRAS G12C mutation. The FDA’s decision on the NDA is expected by Dec 14, 2022.
If adagrasib can outperform any potential competitor from the same class, it will be a huge boost to the stock and can also attract promising buyout offers. Yet, the FDA’s longer-than-expected review period for the NDA was a setback for the company.
This longer period is likely to benefit the drug’s direct competitor
Amgen
AMGN
, which received FDA approval for its own KRAS G12C inhibitor, Lumakras, in second-line NSCLC in 2021. Lumakras generated $90 million in revenues in full-year 2021 for Amgen. A longer review period is likely to benefit Amgen to penetrate more into the market and raise competition. In fact, Amgen received approval for Lumakaras in the European Union in January 2022.
The KRYSTAL-1 study is also evaluating adagrasib in multiple cohorts in combination with other therapies. These include a combination of adagrasib with
Merck
’s
MRK
Keytruda in NSCLC, a combination of adagrasib plus Boehringer Ingelheim’s Gilotrif (afatinib) in advanced NSCLC and adagrasib with
Eli Lilly
’s
LLY
Erbitux in colorectal cancer (“CRC”). Apart from these combinations, Mirati Therapeutics is also pursuing a broad combination development program for adagrasib with SHP2, SOS1 or CDK 4/6 inhibitors.
Please note that while Merck’s Keytruda has been approved by the FDA for multiple cancer indications, Eli Lilly’s Erbitux is currently approved for CRC as well as head and neck cancer indications. For the full year of 2021, Lilly recorded $548.3 million from Erbitux sales, while Merck recorded $17.2 billion as revenues from Keytruda sales.
Shares of Mirati have plunged 42.3% so far this year in comparison with the
industry
’s 17.8% decline.
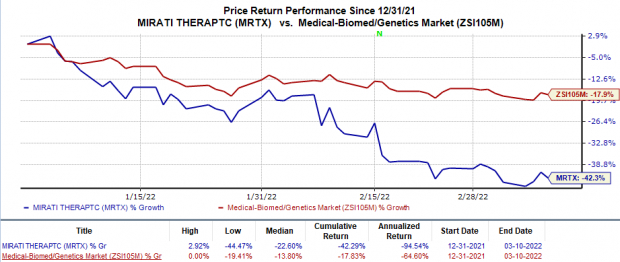
Image Source: Zacks Investment Research
The company’s second pipeline candidate, sitravatinib is designed to selectively target a spectrum of tyrosine kinases, which are involved in both tumor growth and the suppression of immune responses to tumors. The candidate is being evaluated in several studies in combination with checkpoint inhibitor therapies as a treatment for patients who are refractory to prior immune checkpoint inhibitor treatment.
Sitravatinib is being evaluated in a pivotal phase III SAPPHIRE study in combination with Bristol Myers’ Opdivo for second-line or third-line non-squamous NSCLC. Data from the study is expected in second-half 2022. If the data from this study is positive, it could be the basis for regulatory submissions for sitravatinib in the United States and Europe.
Apart from adagrasib and sitravatinib, Mirati initiated a phase I/II study in first-quarter 2022 to evaluate its PRMT5 inhibitor candidate, MRTX1719, as a potential treatment for methylthioadenosine phosphoylase-deleted cancers.
Zacks Rank
Mirati currently carries a Zacks Rank #4 (Sell).
You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2022?
From inception in 2012 through 2021, the
Zacks Top 10 Stocks
portfolios gained an impressive +1,001.2% versus the S&P 500’s +348.7%. Now our Director of Research has combed through 4,000 companies covered by the Zacks Rank and has handpicked the best 10 tickers to buy and hold. Don’t miss your chance to get in…because the sooner you do, the more upside you stand to grab.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









