Sanofi
SNY
announced that it has inked a strategic, risk-sharing collaboration with a private, global investment platform of
Blackstone
BX
, Blackstone Life Sciences, to support the development of the subcutaneous formulation of Sanofi’s myeloma drug, Sarclisa.
Per the agreement, Blackstone Life Sciences will contribute up to €300 million (approximately $330 million), which will be used to accelerate the global pivotal studies and the clinical development program for subcutaneous Sarclisa to treat multiple myeloma (“MM”) patients. Blackstone Life Sciences is eligible to receive royalties on the future sales of subcutaneous Sarclisa, following the successful development and potential approval.
Sanofi is planning to start a pivotal study to evaluate subcutaneous Sarclisa in MM patients in the second half of 2022. The company has partnered with drug delivery technology innovator company, Enable Injections for the subcutaneous formulation delivery of Sarclisa.
We note that the subcutaneous formulation of Sarclisa is not yet approved anywhere. However, Sarclisa is approved as an intravenous injection for treating MM patients. The successful development of subcutaneous Sarclisa will be beneficial for patients for its convenience in administration compared to the intravenous injection.
Sanofi stock has gained 3.4% this year so far against a decrease of 1.9% for the
industry
.
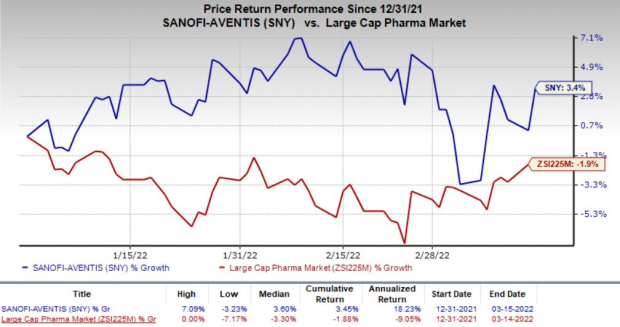
Image Source: Zacks Investment Research
Sarclisa received its first FDA approval in combination with
Bristol-Myers
’
BMY
Pomalyst (pomalidomide) and dexamethasone for treating adults with relapsed/refractory MM having received at least two prior therapies including Revlimid (lenalidomide) and a proteasome inhibitor in 2020.
In December 2021, Sanofi
announced
that a phase III study evaluating Sarclisa in combination with standard-of-care treatment, Bristol-Myers’ Revlimid, bortezomib and dexamethasone in MM patients met its primary endpoint. The combination regimen achieved minimal residual disease negativity in transplant-eligible patients with newly diagnosed MM.
We note that Bristol-Myers’ Pomalyst and Revlimid are both approved for treating MM.
Last year, Sanofi gained FDA approval for the label expansion of Sarclisa for the treatment of adult patients with relapsed/refractory MM in the second- to fourth-line setting in combination with
Amgen
’s
AMGN
Kyprolis (carfilzomib) and dexamethasone.
Kyprolis is a key drug in Amgen’s oncology portfolio approved for multiple myeloma. Amgen is evaluating Kyprolis for additional indications. Kyprolis is being investigated for weekly dosing in combinations with lenalidomide and dexamethasone for relapsed multiple myeloma.
Zacks Rank
Sanofi currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









