Shares of
Moderna, Inc.
MRNA
were up 6.3% after the company announced that it has filed a regulatory application with the FDA, seeking emergency authorization to use a second booster dose of its COVID vaccine in adults, aged 18 years and above.
The regulatory application filed by MRNA seeks to amend Moderna’s existing emergency use authorization (EUA) to allow a fourth dose of its COVID vaccine in adults who already received an initial booster dose of any authorized COVID vaccine in the United States.
Shares of Moderna have declined 29.6% in the year so far compared with with the
industry
’s 12.6% decrease.
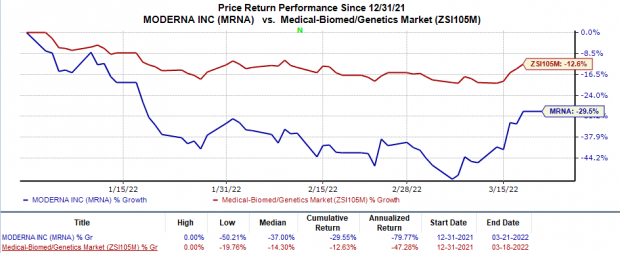
Image Source: Zacks Investment Research
Moderna’s filing with the FDA is based on data generated in the United States and Israel following the emergence of the Omicron variant of the coronavirus. While cases of COVID infection in the United States and many parts of the globe have waned in the past few months, the recent reports of rising infection rates in China and Europe due to the emergence of a new variant called Deltacron re-ignited demand for COVID-19 vaccines.
A rise in COVID-19 cases is likely to drive demand for vaccines and booster doses. Another wave of COVID-19 infection across the globe may lead to ramped-up COVID-19 vaccination programs in different countries.
Last week,
Pfizer
PFE
and partner
BioNTech
BNTX
also submitted a regulatory application with the FDA seeking EUA for a second booster dose of their COVID vaccine, Comirnaty. However, unlike Moderna’s submission, the Pfizer/BioNTech’s application seeks authorization in older adults, aged 65 years and above.
Per Moderna, the reason for seeking approval in the age bracket of 18 years and above is to provide flexibility for healthcare authorities to determine the appropriate use of the second booster dose of its COVID-19 vaccine in relevant patient groups.
We remind investors that the COVID-19 vaccines developed by both Moderna and Pfizer/BioNTech are based on mRNA technology. However, Comirnaty has an edge over MRNA’s COVID vaccine. While the primary regimen of Pfizer/BioNTech’s Comirnaty is authorized for use in children and adolescents in the United States, the third/booster dose of this vaccine is authorized for use in individuals, aged 12 years and above. MRNA is yet to gain authorization for use of its COVID vaccine in individuals under 18 years of age.
Zacks Rank & Stock to Consider
Moderna currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the same sector is
Vertex Pharmaceuticals
VRTX
, which carries a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Vertex Pharmaceuticals’ earnings per share estimates for 2022 have increased from $14.33 to $14.52 in the past 30 days. Shares of VRTX have risen 13.8% year to date.
Earnings of Vertex Pharmaceuticals beat estimates in each of the last four quarters, the average being 10%.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $2.4 trillion by 2028 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Recommendations from previous editions of this report have produced gains of +205%, +258% and +477%. The stocks in this report could perform even better.
See these 7 breakthrough stocks now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









