Ligand Pharmaceuticals
LGND
announced that it has entered into a definitive merger agreement with a publicly traded special purpose acquisition company (“SPAC”),
Avista Public Acquisition Corp. II
AHPA
for the completion of the previously announced spin-off of its antibody discovery business, OmniAb business.
The spin-off of the OmniAb business will be immediately followed by the merger of Ligand’s business with a newly formed subsidiary of Avista Public Acquisition Corp. II. The newly formed merged entity will be renamed to OmniAb, Inc. The spun-off OmniAb business will be led by Ligand’s president, Matt Foehr. The transaction is expected to be closed in the second half of 2022. The spun-off OmniAb business will be listed on the Nasdaq Global Markets under the ticker symbol OABI.
Ligand management is planning to distribute 100% of its ownership of OmniAb to its shareholders in a tax-free distribution.
Please note that SPAC, Avista Public Acquisition Corp. II is sponsored by a leading private equity firm focused on the healthcare industry, Avista Capital Partners (Avista). Per the merger agreement, Avista will invest up to $115 million in the newly-formed OmniAb following the completion of the merger. Ligand will contribute another $15 million to the new business following separation. The combined company, OmniAb, will have an initial pre-money equity valuation of $850 million.
Following the completion of the merger of the SPAC and Ligand’s OmniAb business, the new entity is likely to have a minimum of $130 million in gross cash. The combined entity can have gross cash of up to $266 million in the event of no redemptions by APAC shareholders. Ligand’s shareholders are likely to own approximately 75% to 84% of the combined company, depending on redemptions by SPAC shareholders.
Ligand’s shares have declined 28.9% so far this year compared with the
industry
’s 12.3% decrease.
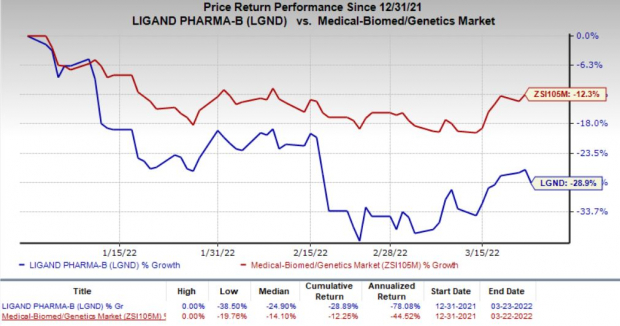
Image Source: Zacks Investment Research
In November 2021, Ligand announced its plan to split its business into two publicly traded companies. One company will handle its OmniAb business along with Ab Initio antigen design technology, Icagen’s ion channel technology and the xPloration high-throughput screening technology. The entity is likely to be named OmniAb, Inc., which will be made public through an initial public offering.
The other entity will own the rest of the business, including the existing collection of core royalties and the technologies, pipeline and contracts associated with the Pelican protein expression platform and the Captisol business.
Ligand believes that the spin-off of its business into two separate entities will likely boost operational focus and business-specific capital allocation. The separation will also likely help to meet partner needs quickly.
Ligand’s Captisol business is already generating revenues as multiple drugs, developed using Captisol, have been approved by the FDA and other regulatory authorities in different countries. Ligand earns royalties on the sales of these drugs, including
Amgen
’s
AMGN
blockbuster drug, Kyprolis.
Amgen’s Kyprolis is approved for treating multiple myeloma. Amgen recorded sales of $1.1 billion in 2021, reflecting year-over-year growth of almost 4%.
The OmniAb business also holds strong potential for growth with diverse antibody repertoires and high-throughput screening technologies that help its partners to discover next-generation therapeutics.
We note that the first OmniAb-derived partnered drug received regulatory approval during the third quarter of 2021. In September, Ligand’s partner, Gloria Biosciences received approval for OmniAb-derived anti-PD-1 monoclonal antibody, zimberelimab, for the treatment of recurrent or refractory classical Hodgkin’s lymphoma in China. During the fourth quarter, Ligand’s partner CStone Pharmaceuticals received approval for an OmniAb-derived anti-PD-L1 monoclonal antibody, Celjemy (sugemalimab), for the first-line treatment of advanced non-small-cell lung cancer (NSCLC) in China.
In December last year, Ligand’s other partner,
J&J
JNJ
, submitted a biologics license application (“BLA”) to the FDA for the approval of its OmniAb-derived candidate, teclistamab. J&J is seeking approval for teclistamab as a treatment for patients with relapsed/refractory multiple myeloma. Ligand is eligible to receive $25 million from J&J as milestone payments upon the first commercial sale of teclistamab.
Currently, 19 different OmniAb-derived antibodies are under development through different partners. A few potential approvals for the partnered OmniAb-derived drugs are expected in the upcoming quarters. Management believes that the platform can generate $500 million to $1 billion of annual royalties beginning 2030.
Zacks Rank
Currently, Ligand is a Zacks Rank #3 (Hold) stock. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top buy-and-hold tickers for the entirety of 2022?
Last year’s 2021
Zacks Top 10 Stocks
portfolio returned gains as high as +147.7%. Now a brand-new portfolio has been handpicked from over 4,000 companies covered by the Zacks Rank. Don’t miss your chance to get in on these long-term buys
Access Zacks Top 10 Stocks for 2022 today >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









