Editas
Medicine, Inc.
EDIT
announced that it has dosed the first pediatric patient in the phase I/II BRILLIANCE study evaluating its lead candidate, EDIT-101, for the treatment of blindness due to Leber congenital amaurosis 10 (LCA10).
The BRILLIANCE study is evaluating the safety of EDIT-101 for the treatment of LCA10 — a rare genetic illness that causes blindness. Currently, there is no therapy approved for treating LCA10.
The company plans to complete dosing in the pediatric mid-dose cohort of the BRILLIANCE study in the first half of 2022. Dosing in the pediatric high-dose cohort is expected to begin later this year.
Shares of Editas were down 4.3% on Monday following the announcement of the news. The stock has plunged 33.9% so far this year compared with the
industry
’s decline of 12.1%.
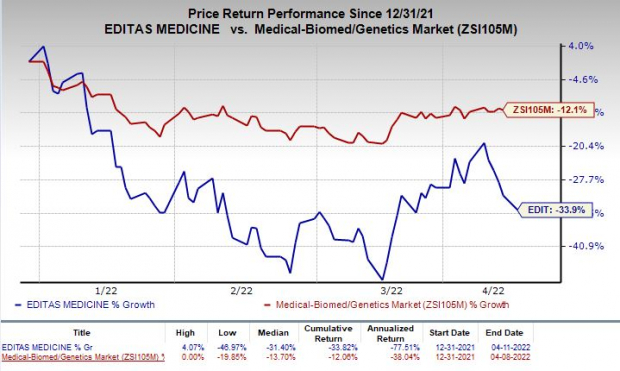
Image Source: Zacks Investment Research
Last June, Editas
started
enrollment in the first of two planned pediatric cohorts in the phase I/II BRILLIANCE study evaluating EDIT-101 for treating LCA10.
In September 2021, Editas
announced
initial data from the phase I/II BRILLIANCE study evaluating EDIT-101 for the treatment of blindness due to LCA10. Early observations from subjects treated in the mid-dose cohort of the study demonstrated clinical evidence of gene editing led by visual improvements.
An update from the BRILLIANCE study is expected in the second half of 2022.
This apart, Editas is evaluating the safety and efficacy of another pipeline candidate, EDIT-301, for treating sickle cell disease.
In December 2021, the FDA cleared the investigational new drug application for EDIT-301 to treat transfusion-dependent beta thalassemia (“TDT”). The company plans to begin a phase I/II study to evaluate the safety, tolerability and preliminary efficacy of EDIT-301 for treating TDT patients. Dosing in the study is expected to begin later in 2022.
Editas has no approved product in its portfolio at the moment. Therefore, pipeline development remains in key focus for the company.
Zacks Rank & Stocks to Consider
Editas currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector are
Galera Therapeutics, Inc.
GRTX
,
Applied Therapeutics, Inc.
APLT
and
Voyager Therapeutics, Inc.
VYGR
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
The Zacks Consensus Estimate for Galera Therapeutics’ loss per share has narrowed 30.6% for 2022 over the past 60 days.
Earnings of GRTX surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions.
Applied Therapeutics’ loss per share estimates have narrowed 11.9% for 2022 over the past 60 days.
Earnings of Applied Therapeutics have surpassed estimates in two of the trailing four quarters, met the same once and missed the same on the other occasion.
Voyager Therapeutics’ loss per share estimates have narrowed 38.6% for 2022 over the past 60 days. The VYGR stock has skyrocketed 203.7% year to date.
Earnings of Voyager Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +25.4% per year. So be sure to give these hand-picked 7 your immediate attention.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








