Coronavirus vaccine stocks may not be the hottest stocks in the
stock market today
. However, the companies behind them continue to play crucial roles in the fight against Covid-19 now. By and large, this is the case as new variants continue to emerge that are boasting greater infectivity. Despite all of this,
coronavirus stocks
have mostly cooled off since their 52-week highs last year. At face value, most would attribute this to the gradual return to normalcy in certain parts of the world. Even so, current data has and continues to point toward a much longer battle against the Covid-19 endemic.
For starters, some of the top U.S. health regulators are already recommending a fourth vaccine dose for older adults. The reason for this is the ongoing surge in new infections due to the Omicron variant of the virus. Just last month, the U.S. FDA greenlit a fourth dose of
Pfizer
(
NYSE: PFE
) and
BioNTech’s
(
NASDAQ: BNTX
) vaccine for individuals aged 50 and older. Speaking of Pfizer, the company is making progress on its oral treatment for “long COVID”, Paxlovid. According to the company, one of the trial participants for Paxlovid as a long COVID treatment saw notable improvements from her symptoms on Day-3 of a five-day course.
For those uninitiated, long COVID is a condition where individuals experience a variety of symptoms after recovering from the initial infection. These symptoms range from fatigue and chest pain to even brain fog, lasting more than three months. Overall, Pfizer estimates it is currently impacting 100 million people globally. Nevertheless, while the world attempts to live with Covid-19, coronavirus vaccine firms are still hard at work. With that said, could one of these three be worth watching in the
stock market
now?
Coronavirus Vaccine Stocks To Buy [Or Sell] Now
-
Moderna Inc.
(
NASDAQ: MRNA
) -
Johnson & Johnson
(
NYSE: JNJ
) -
Novavax Inc.
(
NASDAQ: NVAX
)
Moderna Inc.
Starting us off today, we have
Moderna
, a company that continues to advance programs in the field of messenger RNA (mRNA), to an enterprise with a diverse clinical portfolio of vaccines. The company also has a broad intellectual property portfolio in areas including mRNA and lipid nanoparticle formulation. It also boasts an integrated manufacturing plant that allows for both clinical and commercial production at scale. Last week, the company announced that the first participants have been dose in the Phase 1/2 study of its company’s seasonal influenza vaccine candidates, mRNA-1020 and mRNA-1030.
This Phase 1/2 randomized, observer-blind, dose-ranging study will evaluate the safety, reactogenicity and immunogenicity of a single dose of mRNA-1020 or mRNA-1030 in healthy adults 18 years and older in the U.S.
“We are pleased to apply Moderna’s mRNA platform to address the longstanding design and manufacturing challenges associated with developing seasonal influenza vaccines. We believe that by targeting both hemagglutinin and neuraminidase, we can achieve broader immunity and higher vaccine efficacy against circulating influenza strains than traditional influenza vaccines. Moreover, we expect that our platform’s flexibility in targeting multiple strains coupled with our ability to manufacture quickly will facilitate production of a vaccine that matches the predominant circulating influenza strain,”
said Stéphane Bancel, Chief Executive Officer of Moderna.
The company will also be announcing its first-quarter financials for 2022 on May 4, 2022 before the market opens. Recently, Taiwan’s Food and Drug Administration says that it has provided authorization for Moderna’s vaccine for children aged six to eleven. The company also has many vaccines in its development pipeline that include the common flu and respiratory virus RSV. With this piece of information, should you invest in MRNA stock?
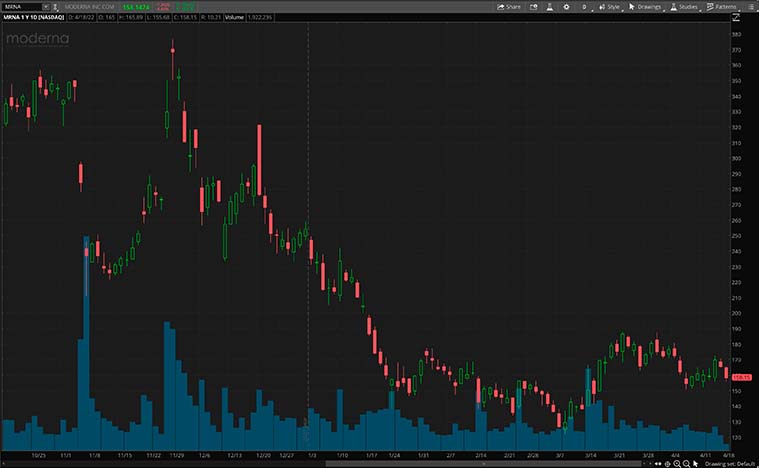
[Read More]
Stock Market Today: Dow Jones, S&P 500 Edged Lower; Twitter In The Spotlight On Musk’s Possible Tender Offer
Johnson & Johnson
Another coronavirus vaccine maker to consider now would be
Johnson & Johnson
, or JNJ, for short. In brief, it is the world’s largest and most broadly-based health care firm. As most would know, the company offers a vast array of day-to-day health care offerings worldwide. From consumer health and pharmaceuticals to biotech, JNJ does it all.
Accordingly, JNJ is in focus now following recent data from the U.S. Centers for Disease Control and Prevention (CDC). Namely, the CDC’s one-year review of JNJ’s vector-based vaccine suggests that it is holding up against its top rivals. In other words, the shot is just as effective at preventing infections, hospitalizations, and deaths as Pfizer’s and Moderna’s. Additionally, this also appears to be the case in terms of breakthrough infectivity as well. The CDC’s data suggests that breakthrough Covid-19 cases in JNJ shot recipients currently stand at 18.8 per 100,000.
For a sense of scale, the CDC’s study covers a sizable U.S.-based sample. This includes JNJ shot recipients aged 5 and above from across all regions in the U.S. As it stands, the agency’s sampling reportedly represents more than 60% of the population. As such, would you consider JNJ stock worth investing in for the long run?
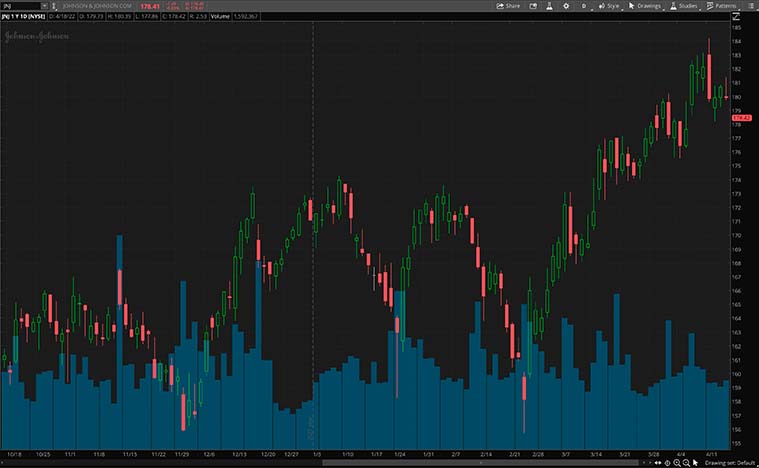
[Read More]
Top Stock Market News For Today April 18, 2022
Novavax Inc.
Novavax
is a clinical-stage vaccine company that continues to deliver novel products to prevent a broad range of infectious diseases. From respiratory diseases and beyond, its vaccine and adjuvant technology can be applied to address both current and emerging global health threats. Also, its recombinant nanoparticle vaccine technology allows the company to combine both the power and speed of genetic engineering to efficiently produce protein-based nanoparticles.
Worth mentioning, the Japanese Health Ministry (JHM) committee has approved Novavax’s Covid-19 vaccine. In turn, the Japanese government is now planning to purchase 150 million doses of the shot. The likes of Novavax will manufacture locally via the Takeda Pharmaceutical Company. Following this approval, the JHM is now looking towards clearing a fourth vaccine shot for the virus in Japan as well. All in all, this would be a solid step forward for Novavax.
Last week, the company also announced that Swissmedic, the Swiss Agency for Therapeutic Products, has granted Novavax conditional marketing authorization (CMA) for Nuvaxovid for active immunization to prevent Covid-19 in individuals 18 years of age and older. It will also be the first protein-based vaccine to be authorized for use in Switzerland. All things considered, is NVAX stock a buy?
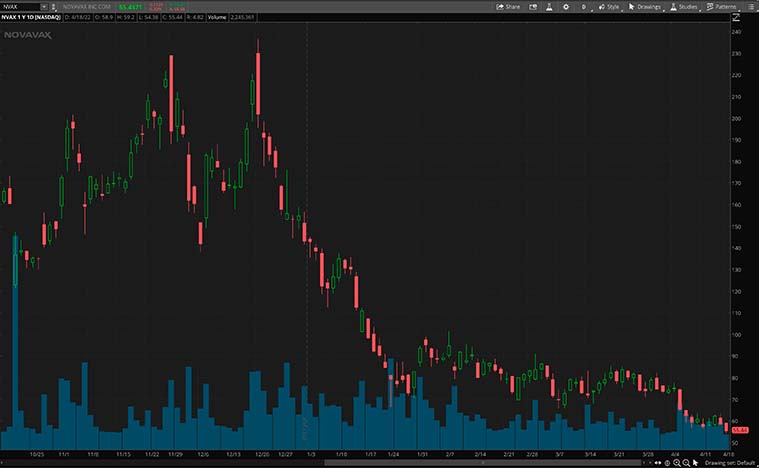
If you enjoyed this article and you’re interested in learning how to trade so you can have the best chance to profit consistently then you need to checkout this YouTube channel.
CLICK HERE RIGHT NOW!!








