Shares of
Prothena Corporation
PRTA
have gained 50.6% in the past year against the
industry
’s decline of 32.7%.
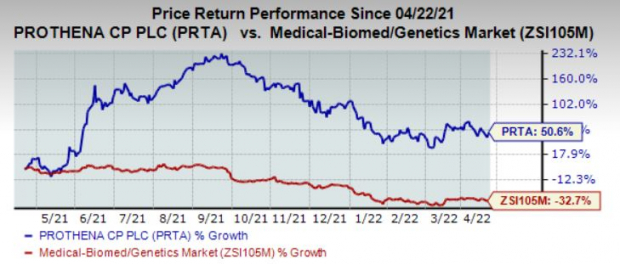
Image Source: Zacks Investment Research
The FDA’s approval of
Biogen’s
BIIB
Alzheimer’s disease (AD) drug, Aduhelm, in 2021 put the spotlight on other companies that are developing AD drugs as investors are now optimistic about the prospects of these pipeline candidates. Prothena is one of them.
Prothena’s AD portfolio spans next-generation antibody immunotherapy, small molecule and vaccines.
The FDA recently cleared the investigational new drug (IND) application for Prothena’s PRX012, a potential best-in-class anti-amyloid beta (Aβ) antibody in development to treat AD. Prothena has initiated the phase I single ascending dose (SAD) study to investigate the safety, tolerability, immunogenicity and pharmacokinetics of PRX012 in both healthy volunteers and patients with AD.
The company expects to initiate the phase I multiple ascending dose study by year-end 2022.
Prothena is advancing an early-stage pipeline of programs for a number of potential neurological indications with
Bristol Myers
BMY
. This includes PRX005 — a potential treatment for AD — an investigational antibody that targets tau, a protein implicated in diseases including AD, frontotemporal dementia, progressive supranuclear palsy, chronic traumatic encephalopathy and other tauopathies. The company has received an $80 million option payment from Bristol Myers to execute the U.S. license agreement in 2021. A phase I study was initiated in 2021, and top-line data is expected in 2022.
The company is also developing a dual Aβ-Tau vaccine — a potential prevention and treatment for AD — to target key epitopes within Aβ and tau proteins to promote amyloid clearance and blockade pathogenic tau interaction. An IND for the vaccine is anticipated in 2023.
Another candidate in Prothena’s pipeline is prasinezumab, which is being developed in collaboration with
Roche
RHHBY
for Parkinson’s disease.
Prothena earned a $60 million clinical milestone payment in 2021 from Roche upon dosing the first patient in the global phase IIb PADOVA study for prasinezumab.
It is also evaluating birtamimab, a potential treatment for AL amyloidosis. It reached a Special Protocol Assessment agreement with FDA and initiated a confirmatory phase III AFFIRM-AL study of birtamimab in Mayo Stage IV patients with AL amyloidosis in 2021.
The successful development and commercialization of the candidates will be a big boost for the company and should propel growth.
However, the uptake of Biogen’s Aduhelm has been slow due to limited patient access amid a lack of clarity on the drug’s reimbursement. Hence, there is a long way to go for companies like Prothena in the AD space. Further, the lack of any approved product in the portfolio is a deterrent.
Prothena currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








