Iovance Biotherapeutics, Inc.
IOVA
incurred a loss of 58 cents per share for first-quarter 2022, narrower than the Zacks Consensus Estimate of a loss of 64 cents but wider than the year-ago loss of 51 cents.
In the absence of any marketed product and revenue-generating collaboration, the company did not record any revenues during the quarter.
Shares of Iovance have declined 22.3% so far this year in comparison with the
industry
’s 20.1% decrease.
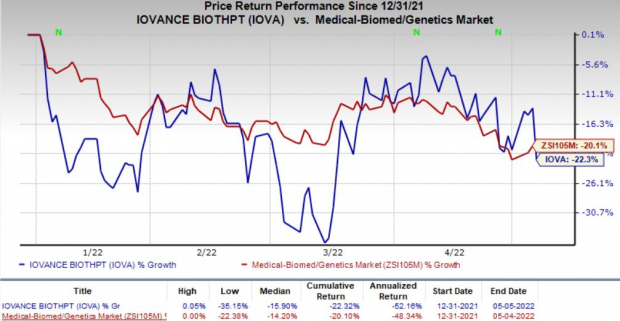
Image Source: Zacks Investment Research
Quarter in Detail
Research & development expenses were $68.3 million, 22.2% higher than the year-ago quarter, primarily due to an increase in related personnel costs and facility building costs.
General and administrative expenses increased 19.4% from the prior-year quarter to $23.4 million due to an increase in related personnel costs, facility-related costs, and intellectual property filing and legal expenses.
The company had $516 million in cash, cash equivalents, short-term investments and restricted cash as of Mar 31, 2022 compared with $602.1 million on Dec 31, 2021.
It expects the cash level to be sufficient to fund the current and planned operations in 2024.
Pipeline Updates
Iovance is developing its lead pipeline candidate, lifileucel, as a monotherapy for treating metastatic melanoma and metastatic cervical cancer in separate pivotal phase II studies — C-144-01 and C-145-04 — for metastatic melanoma and recurrent, metastatic or persistent cervical cancer, respectively, in previously-treated patients.
In April, the FDA provided
positive feedback
regarding the potency assays and assay matrix for lifileucel, concerning the treatment of melanoma. The regulatory authority had previously raised concerns over the potency assays in 2020. The resolution of the potency assay-related concern was necessary prior to the submission of a biologics license application (BLA) for lifileucel.
Following the positive feedback from the FDA, Iovance is planning to request a pre-BLA meeting in July followed by the BLA submission in August.
Iovance is currently in discussions with the FDA related to a BLA for lifileucel as a cervical cancer therapy. The company will design its registrational strategy for cervical cancer following an FDA dialogue and feedback.
The company is developing lifileucel in combination regimens with other cancer therapies. A multi-cohort phase II study — IOV-COM-202 — is evaluating lifileucel in combination with
Merck
’s
MRK
immunotherapy, Keytruda (pembrolizumab). Updated data from a cohort of the mid-stage study demonstrated that lifileucel plus Merck’s Keytruda led to an overall response rate (ORR) of 67% in metastatic melanoma patients who did not receive any treatment with anti-PD-1 therapies like MRK’s Keytruda. Iovance plans to start a separate phase III study to evaluate the combination regimen as frontline metastatic melanoma treatment in late 2022.
Iovance is also evaluating another tumor-infiltrating lymphocyte (TIL) therapy, LN-145, as a monotherapy for head and neck squamous cell carcinoma and non-small cell lung cancer (NSCLC) in two separate studies.
The company is also evaluating LN-145 in combination with Merck’s Keytruda or
Bristol-Myers
’
BMY
Opdivo/Yervoy in different cohorts of IOV-COM-202. The study is evaluating LN-145 plus Keytruda or BMY’s Opdivo/Yervoy in separate cohorts in NSCLC patients.
Both Opdivo and Yervoy are two blockbuster immunotherapies from Bristol-Myers.
In April, Iovance received FDA approval to start an early-stage clinical study to evaluate its new TIL candidate, IOV-4001, in patients with advanced melanoma or metastatic NSCLC. A study is
expected to begin
later this year.
IOVA is also progressing well with its IND-enabling studies including IOV-3001, a novel IL-2 analog. The company gained a license for the development and commercialization of IOV-3001 from
Novartis
NVS
in 2020.
Per the agreement with Novartis, Iovance paid an upfront payment and may also pay milestone payments. NVS is also eligible for royalties on any potential sales from IOV-3001.
Zacks Rank
Iovance currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +25.4% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









