Novavax, Inc.
NVAX
reported earnings of $2.56 per share for first-quarter 2022, which missed the Zacks Consensus Estimate of $3.33. In the year-ago quarter, NVAX had posted a loss of $3.05 per share. This quarter under review is NVAX’s first profitable quarter.
Revenues for the quarter were $704 million, up 57.4% year over year. Revenues missed the Zacks Consensus Estimate of $878.1 million.
Shares of Novavax were down 22.3% in after-hours trading on Monday, following its worse-than-expected results. The stock has plunged 69.7% so far this year compared with the
industry
’s 28% decrease.
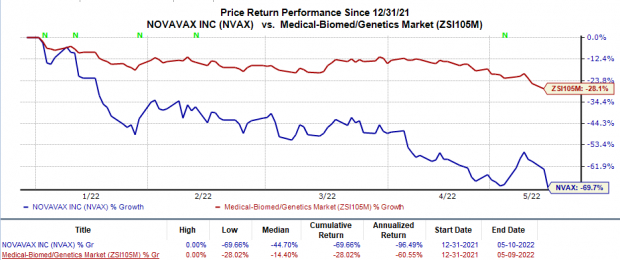
Image Source: Zacks Investment Research
Quarter in Detail
During the first quarter, NVAX generated $586.0 million as product sales from NVX-CoV2373, its protein-based COVID-19 vaccine. This quarter is the first time when Novavax recorded revenues from its product sales. NVAX also recorded $19.0 million of revenues from royalties and adjuvant sales to license partners.
Novavax’s grant revenues declined 77.8% year over year to $99 million for the same period as it materially completed the activities required under the agreements with the U.S. Government.
For the reported quarter, research and development expenses were $383 million, down 35.3% year over year. This decline was attributable to lower clinical development activities for NVX-CoV2373 as well as lower manufacturing costs for the vaccine incurred during the quarter.
General and administrative expenses rose 51.9% year over year to $96 million. The rise in expenses was on account of activities to support the commercial launch of NVX-CoV2373.
As of Mar 31, 2022, Novavax had $1.6 billion of cash and cash equivalents compared with $1.5 billion as of Dec 31, 2021.
2022 Guidance
Novavax maintained its previously issued 2022 revenue guidance between $4 billion and $5 billion.
Recent Updates
In the first quarter, Novavax received authorization for use of its COVID-19 vaccine in adults in markets like Australia, Bangladesh, Canada, Great Britain and Thailand.
After multiple delays, Novavax finally
filed
for Emergency Use Authorization of its COVID-19 vaccine with the FDA in January. An opinion of the FDA’s Vaccines And Related Biological Products Advisory Committee (VRBPAC) on this filing is expected by next month. Regulatory filing seeking authorization for use of NVX-CoV2373 is also filed in South Africa.
Novavax is also evaluating the vaccine across multiple studies. Earlier in February 2022, NVAX
reported
positive data from the pediatric expansion of the phase III PREVENT study, which evaluated its COVID-19 vaccine in adolescents aged between 12 years and 17 years of age.The study achieved its primary effectiveness endpoint of NVX-CoV2373, generating neutralizing antibodies in adolescents, similar to the antibody responses in young adult patients (aged between 18 years and 26 years) who were administered the vaccine in the phase III PREVENT study.
In March 2022, NVAX received the first authorization for the use of its COVID vaccine in adolescents in India. Novavaxalso filed regulatory applications seeking authorization for use of its COVID vaccine in adolescents in Australia, Europe and New Zealand.
NVAX is also evaluating a COVID-19-influenza combination (CIC) vaccine candidate in a phase I/II study. The CIC vaccine is a combination of NVX-CoV2373 and NanoFlu in a single formulation. Last month, Novavax
announced
initial data from this study, which demonstrated the CIC vaccine to be feasible and immunogenic. The immune responses were comparable to the standalone NanoFlu and the standalone NVX-CoV2373 formulations. NVAX expects to begin a phase II dose confirmation study by this year-end.
Novavax is also advancing the clinical development of booster studies of NVX-CoV2373. Last December, NVAX initiated dosing patients in an extension of the phase III PREVENT-19 study to evaluate the third/booster dose of NVX-CoV2373. In April 2022, NVAX received the first approval for use of a booster dose of NVX-CoV2373 in adults in Japan. NVAX is also presently evaluating the booster dose of the vaccine in a late-stage study in adolescents.
NVAX expects to report top-line data during third-quarter 2022 from the clinical studies evaluating its Omicron-specifc booster vaccine.
If authorized in the United States, Novavax’s COVID vaccine will face stiff competition from the COVID vaccines developed by
Moderna
MRNA
and
Pfizer
PFE
/
BioNTech
BNTX
, which currently dominate the U.S. market. In fact, the vaccines developed by Pfizer/BioNTech and Moderna are currently the only ones that received full approval in the United States.
The vaccines developed by Moderna and Pfizer/BioNTech are based on the mRNA technology and require a two-dose primary regimen. Two booster doses of Moderna and Pfizer/BioNTech vaccines are also authorized for use in the United States.
Zacks Rank
Currently, Novavax has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Bitcoin, Like the Internet Itself, Could Change Everything
Blockchain and cryptocurrency has sparked one of the most exciting discussion topics of a generation. Some call it the “Internet of Money” and predict it could change the way money works forever. If true, it could do to banks what Netflix did to Blockbuster and Amazon did to Sears. Experts agree we’re still in the early stages of this technology, and as it grows, it will create several investing opportunities.
Zacks’ has just revealed 3 companies that can help investors capitalize on the explosive profit potential of Bitcoin and the other cryptocurrencies with significantly less volatility than buying them directly.
See 3 crypto-related stocks now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









