Gilead Sciences, Inc
.
GILD
announced that the FDA has lifted the clinical hold placed on its investigational new drug application (IND) to evaluate injectable lenacapavir for HIV treatment and HIV pre-exposure prophylaxis (PrEP).
Lenacapavir is Gilead’s potential first-in-class, investigational long-acting HIV-1 capsid inhibitor in development for the treatment and prevention of HIV-1 infection.
In December 2021, the FDA placed a clinical hold on the use of injectable lenacapavir in borosilicate vials in all ongoing clinical studies for HIV treatment and HIV PrEP.
The hold was due to emerging concerns about the compatibility of vials made of borosilicate glass with lenacapavir solution, which could potentially lead to the formation of sub-visible glass particles in the solution of lenacapavir.
The FDA took the decision following its review of Gilead’s comprehensive plan and corresponding data on the storage and compatibility of lenacapavir injection with an alternative vial made from aluminosilicate glass.
Consequently, screening and enrollment of study participants and the dosing of injectable lenacapavir can continue.
We remind investors that the FDA granted Breakthrough Therapy Designation to develop lenacapavir to treat HIV infection in heavily treatment-experienced patients with multi-drug resistance in combination with other antiretroviral drugs in 2019.
However, the FDA has issued a complete response letter (CRL) for its new drug application (NDA) in this population due to vial compatibility.
The safety, efficacy and dosing of lenacapavir are being evaluated in multiple ongoing clinical studies, including CAPELLA, a phase II/III, double-blinded, placebo-controlled global multicenter study. The study is designed to evaluate the antiviral activity of lenacapavir administered every six months as a subcutaneous injection in heavily treatment-experienced people with multi-drug resistant HIV-1 infection.
Gilead’s stock has lost 14.3% so far in the year compared with the
industry
‘s decline of 25%.
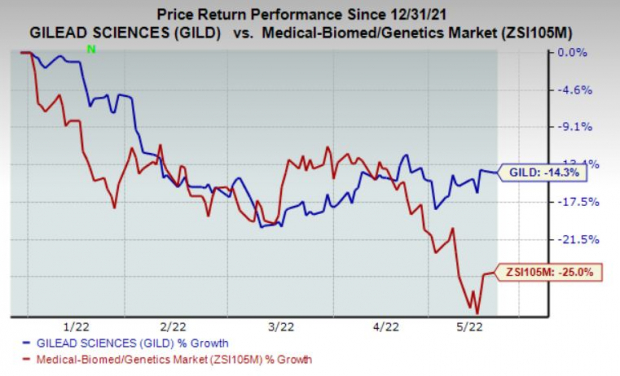
Image Source: Zacks Investment Research
The HIV franchise is the lead growth driver for Gilead propelled by the solid uptake of Biktravy.
Concurrently, Gilead is making efforts to develop its oncology business to diversify its revenue base as competition is stiff in the HIV business from the likes of
GlaxoSmithKline
GSK
. Also, the loss of exclusivity for Truvada has hurt the company’s business.
Glaxo’s HIV franchise recorded 14% growth in the quarter. Growth was driven by new HIV products Dovato, Cabenuva, Rukobia, Juluca and Apretude and phasing.
Gilead currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks are
Alkermes
ALKS
and
Geron Corporation
GERN
. While Alkermes sports a Zacks Rank #1 (Strong Buy), Geron has a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank stocks here
.
Loss estimates for ALKS for 2022 have narrowed to 3 cents from a loss of 14 cents in the past 60 days. Alkermes surpassed estimates in all of the trailing four quarters, the average surprise being 350.48%.
Loss estimates for GERN for 2022 have narrowed by 6 cents in the past 60 days. Geron surpassed estimates in three of the trailing four quarters, the average surprise being 1.07%.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2022?
From inception in 2012 through 2021, the
Zacks Top 10 Stocks
portfolios gained an impressive +1,001.2% versus the S&P 500’s +348.7%. Now our Director of Research has combed through 4,000 companies covered by the Zacks Rank and has handpicked the best 10 tickers to buy and hold. Don’t miss your chance to get in…because the sooner you do, the more upside you stand to grab.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report







