AbbVie
ABBV
announced that the FDA has approved its drug Skyrizi (risankizumab) for its third indication, moderately to severely active Crohn’s disease (“CD”).
Skyrizi can now be used as a 600mg intravenous induction at week 0, week 4, and week 8, followed by 360 mg self-administered by subcutaneous injection with an on-body injector at week 12, and every 8 weeks thereafter.
AbbVie’s stock has risen 2.1% this year so far against a decrease of 2.6% for the
industry
.
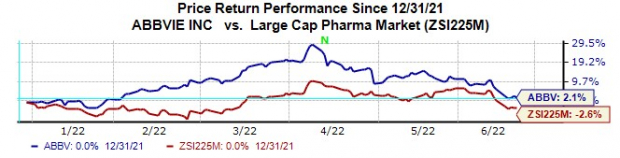
Image Source: Zacks Investment Research
In September last year, the FDA delayed its decision on Skyrizi for the CD indication by three months as it needed time to review the additional information filed by AbbVie, including information about the on-body injector. Skyrizi’s approval for the CD indication was supported by data from two phase III induction studies — ADVANCE, MOTIVATE, and one maintenance study — FORTIFY. In the studies, Skyrizi demonstrated significant improvements in both the co-primary endpoints of endoscopic response and clinical remission as both an induction and maintenance therapy.
Skyrizi, an interleukin-23 (IL-23) inhibitor, is presently approved to treat moderate-to-severe plaque psoriasis and active psoriatic arthritis while being evaluated in phase III studies for psoriasis, ulcerative colitis and psoriatic arthritis. Skyrizi is under review in Europe for the CD indication.
AbbVie is developing Skyrizi in collaboration with Boehringer Ingelheim, with AbbVie leading the global development and commercialization of Skyrizi.
Skyrizi recorded sales of $940 million in the first quarter of 2022, up 65.6% year over year on an operational basis. Skyrizi and AbbVie’s another newer inflammatory drug, Rinvoq have demonstrated differentiated clinical profiles versus AbbVie’s blockbuster medicine, Humira and are already contributing meaningful revenues, including $4.6 billion in combined sales in 2021. Both Skyrizi and Rinvoq were approved for active psoriatic arthritis in 2021/early 2022. Rinvoq was approved for atopic dermatitis, ulcerative colitis and ankylosing spondylitis in the first half of 2022. With such new indications, sales of these drugs could be higher and have the potential to replace Humira when generics are launched in the United States in 2023. AbbVie expects combined sales of Skyrizi and Rinvoq to be more than $15 billion by 2025.
Zacks Rank and Stocks to Consider
AbbVie currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Some better-ranked biotech stocks are
Sesen Bio
SESN
,
Alkermes
ALKS
and
BELLUS Health
BLU
. While Alkermes and Sesen Bio have a Zacks Rank of 1, BELLUS Health has a Zacks Rank of 2 (Buy). You can see
the complete list of today’s Zacks #1 Rank stocks here
.
The Zacks Consensus Estimate for Sesen Bio’s2022 loss has declined from 33 cents to 32 cents per share in the past 60 days. Shares of SESN have risen 8.2% in the past three months.
Earnings of Sesen Bio beat estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 69.9%.
The Zacks Consensus Estimate for Alkermes’ 2022 loss per share has narrowed from 14 cents to 3 cents in the past 60 days. Shares of ALKS have risen 8.3% in the past three months.
Earnings of Alkermes beat estimates in each of the last four quarters, the average being 350.5%.
The Zacks Consensus Estimate for BELLUS Health’s 2022 loss per share has narrowed from 87 cents to 76 cents while that for 2023 has gone down from $1.11 per share to 98 cents per share in the past 60 days. Shares of BLU have risen 23.1% in the past three months.
BELLUS Health delivered a four-quarter average negative earnings surprise of 2.68%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








