Alnylam Pharmaceuticals, Inc.
ALNY
incurred a loss of $2.00 per share in the first quarter of 2022, wider than the Zacks Consensus Estimate of a loss of $1.93. The loss includes stock-based compensation expenses and unrealized gain on equity securities. Excluding these items, adjusted loss was $1.49 per share, narrower than the adjusted loss of $1.64 reported in the year-ago quarter.
The company recorded total revenues of $213.3 million in the quarter, which also missed the Zacks Consensus Estimate of $244 million. In the year-ago quarter, total revenues were $177.6 million. Net product revenues were $186.8 million, up 38% year over year, driven by the global expansion of Onpattro (patisiran) and Givlaari (givosiran), as well as encouraging initial uptake for Oxlumo (lumasiran) following its launch in the first quarter of 2021.
Net revenues from collaborators were $25.9 million, down from $41.7 million in the year-ago quarter, primarily due to a decrease in revenues recognized in connection with the collaboration agreements with
Regeneron
REGN
.
During the first quarter, Alnylam also recorded royalty revenues of $0.4 million, owing to the global sales of Leqvio (inclisiran) from its partner,
Novartis
NVS
.
Novartis has obtained global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam.
Alnylam is entitled to receive tiered royalties on the global sales of Leqvio from Novartis.
Shares of Alnylam were down 7.6% on Thursday following the announcement of weaker-than-expected first-quarter results. The stock has lost 15.6% so far this year compared with the
industry
’s decline of 20.4%.
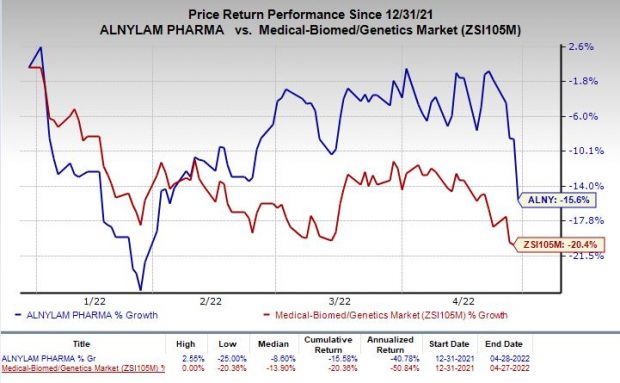
Image Source: Zacks Investment Research
Quarter in Detail
Onpattro is approved for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis. The injection recorded sales of $137 million in the first quarter, up around 34% year over year, driven by new patient demand. Per the company, as of Mar 31, 2022, more than 2,200 patients have received treatment with Onpattro worldwide.
Alnylam’s second product, Givlaari, was approved for the treatment of acute hepatic porphyria in the United States in November 2019 and in Europe in March 2020. In the first quarter of 2022, Givlaari recorded sales of $35.3 million, reflecting an increase of around 43% year over year.
Oxlumo injection for subcutaneous use was approved in November 2020 for the treatment of primary hyperoxaluria type 1 (PH1) to lower urinary oxalate levels in pediatric and adult patients. The injection recorded global net product revenues of about $14.5 million in the first quarter of 2022, reflecting an increase of 59.5% year over year. Oxlumo sales declined 24.4% sequentially.
Adjusted research and development expenses (R&D) decreased to $158.3 million from $161.5 million reported in the year-ago quarter. The decrease was due to lower expenses incurred in clinical studies.
Adjusted selling, general and administrative expenses (SG&A) rose to $136,7 million from $115.5 million incurred in the year-ago quarter. The increase was due to higher legal and other expenses to support strategic growth.
2022 Guidance
Alnylam lowered its net product revenue guidance for 2022. This might have hurt investors’ sentiments and also resulted in the stock going down following the announcement.
The company now expects net product revenues for Onpattro, Givlaari and Oxlumo in the range of $870-$930 million compared with the previous expectation of $900-$1,000 million for 2022.
Net revenues from collaborations and royalties are expected in the range of $175-$225 million, unchanged from the previous view. Adjusted R&D and SG&A expenses are anticipated in the band of $1,390-$1,450 million compared with the previous projection of $1,400-$1,500 million.
Pipeline Updates
In April 2022, the FDA
delayed
its decision on Alnylam’s new drug application (“NDA”) for its investigational RNAi therapeutic, vutrisiran, developed for the treatment of transthyretin-mediated (ATTR) amyloidosis.
A decision from the regulatory body is now expected on Jul 14, 2022, instead of the previously expected date of Apr 14.
In March 2022, FDA accepted the sNDA for Oxlumo (lumasiran) for the reduction of plasma oxalate in the treatment of patients with advanced primary hyperoxaluria type 1. A decision from the regulatory body is expected on Oct 6, 2022.
In December 2021, Alnylam
submitted
a supplemental new drug application (sNDA) to the FDA and a Type II filing variation to the European Medicines Agency for lumasiran for the reduction of plasma oxalate for the treatment of patients with advanced primary hyperoxaluria type 1.
In January 2022, Alnylam began a collaboration with Novartis to explore targeted therapy to restore liver function. The companies have agreed to collaborate on the discovery and development of siRNA-based targeted therapy to restore functional liver cells in patients with end-stage liver disease.
Alnylam, in collaboration with Regeneron, is advancing cemdisiran, an investigational RNAi therapeutic, for the treatment of complement-mediated diseases.
Regeneron has initiated phase III studies of cemdisiran and pozelimab combination for treating myasthenia gravis and paroxysmal nocturnal hemoglobinuria.
Zacks Rank & Key Pick
Alnylam currently carries a Zacks Rank #3 (Hold).
A top-ranked stock in the biotech sector is
BioMarin Pharmaceutical Inc.
BMRN
, sporting a Zacks Rank #1 (Strong Buy) at present. You can see
the complete list of today’s Zacks #1 Rank stocks here
.
BioMarin’s earnings estimates have been revised 1% upward for 2022 and 1.8% for 2023 over the past 60 days.
Earnings of BMRN surpassed estimates in each of the trailing four quarters.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +25.4% per year. So be sure to give these hand-picked 7 your immediate attention.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









