Shares of
Ascendis Pharma A/S
ASND
rose more than 21% on Aug 26 following approval of its biologics license application (BLA) for Skytrofa (TransConhGH; lonapegsomatropin-tcgd) by the FDA. The BLA sought approval for the long-acting growth hormone treatment drug as a treatment for growth failure due to inadequate secretion of endogenous growth hormone (GH) in pediatric patients 1 year or older and who weigh at least 11.5 kg (25.4 lb).
The drug is the first approved product in the company’s portfolio. It is also the first FDA-approved product that delivers growth hormone (somatropin) by sustained release over 1 week. The drug has the potential to replace current standard-of-care of daily somatropin therapies due to its once-weekly administration.
The drug will be available in the United States shortly along with a full suite of patient support programs, including proper injection procedures. Along with the drug, the FDA also approved new Skytrofa Auto-Injector and cartridges which will allow patients to store the medicine at room temperature for up to six months following first removal from a refrigerator. Moreover, the once-weekly administration of Skytrofa will reduce injections per year by up to 86% for patients currently receiving daily injections.
A regulatory application seeking approval for lonapegsomatropin for pediatric growth hormone deficiency (GHD) is under review in Europe. A decision is expected in the fourth quarter of 2021.
Pediatric GHD is a serious orphan disease, which is caused when the pituitary gland does not produce enough growth hormone.
Shares of Ascendis have declined 10.2% so far this year against the
industry
’s increase of 1.3%.
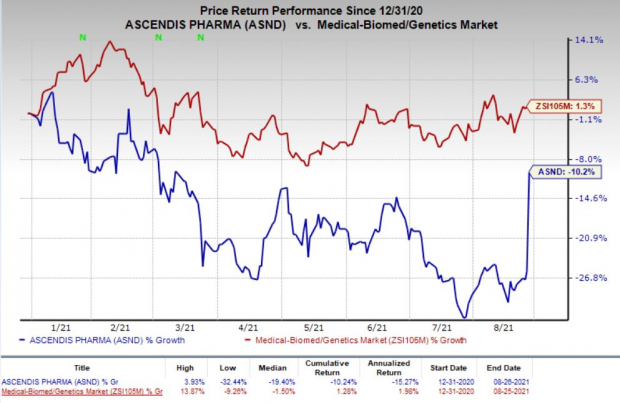
Image Source: Zacks Investment Research
The BLA for Skytrofa was approved by the FDA based on data from the phase III study — heiGHt — that evaluated one-weekly Skytrofa in treatment-naïve children with GHD over 52-weeks for improvement in annualized height velocity (AHV) compared to
Pfizer
’s
PFE
daily somatropin drug, Genotropin. The study met its primary endpoint of non-inferiority in AHV for Skytrofa compared to Genotropin. Data from the study demonstrated that treatment with Skytrofa improved mean AHV by 0.9 cm/year compared to Genotropin. While mean AHV was 11.2 cm/year for Skytrofa, Genotropin achieved mean AHV of 10.3 cm/year over 52 weeks of treatment.
Meanwhile, lonapegsomatropin enjoys Orphan Drug designation for GHD in both the United States and Europe. However, some large as well as small biotechs are also engaged in developing treatment for GHD including
Novo Nordisk
NVO
and
I-Mab Biopharma
IMAB
.
Apart from lonapegsomatropin, the company is also evaluating multiple other pipeline candidates developed using TransCon. Key among them include TransCon CNP and TransCon PTH. A phase II study is evaluating TransCon CNP for the treatment of achondroplasia, the most common form of dwarfism. TransCon PTH is being evaluated in a phase III study as a potential treatment for hypoparathyroidism in adult patients. Top-line data from the late-stage study is expected in the fourth quarter of 2021.
Zacks Rank
Ascendis currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









