AstraZeneca
AZN
and partner Daiichi Sankyo announced that the European Commission has approved their drug Enhertu for previously treated patients with HER2-positive advanced gastric cancer who have received a prior trastuzumab-based regimen. The approval for this expanded use of Enhertu was based on data from the DESTINY-Gastric02 and the DESTINY-Gastric01 phase III studies in which Enhertu demonstrated clinically meaningful efficacy. Following approval of Enhertu for gastric cancer, AstraZeneca will make a milestone payment of $35 million to Daiichi Sankyo
In the United States, Enhertu was approved for advanced or metastatic HER2-positive gastric cancer in January 2021.
AstraZeneca stock has risen 15.8% this year so far compared with an increase of 11% for the
industry
.
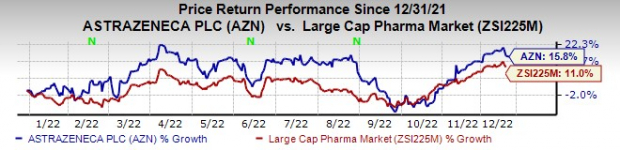
Image Source: Zacks Investment Research
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency recommended expanded use of some of AstraZeneca’s medicines.
The CHMP gave a positive opinion recommending the approval of Enhertu for previously treated patients with HER2-low metastatic breast cancer.
Enhertu is the first-ever HER2-directed medicine to demonstrate a significant survival benefit versus chemotherapy for the treatment of patients with HER2-low metastatic breast cancer. The CHMP’s positive opinion was based on data from the pivotal phase III DESTINY-Breast04 study. In the study, Enhertu reduced the risk of disease progression or death by 50% and increased overall survival by more than six months versus chemotherapy. The FDA approved Enhertu for the same patient population in August.
The CHMP also recommended approving AstraZeneca’s dual immunotherapy regimen, comprising PD-L1 inhibitor Imfinzi plus CTLA-4 antibody Imjudo (tremelimumab), combined with platinum-based chemotherapy, for treating adult patients with Stage IV (metastatic) non-small cell lung cancer (NSCLC). This CHMP recommendation for the Imfinzi+Imjudo combo was based on data from the phase III POSEIDON study. Imfinzi plus Imjudo was approved by the FDA for this patient group in November.
The CHMP also recommended approving Imfinzi in combination with Imjudo for patients with unresectable hepatocellular carcinoma (HCC), the most common type of liver cancer. The approval was based on positive data from the HIMALAYA phase III study. Imfinzi plus Imjudo was approved by the FDA for this patient group in October.
The CHMP recommended expansion of the label of Forxiga for the treatment of symptomatic chronic heart failure (HF) with mildly reduced or preserved ejection fraction. At present, Forxiga is approved in the EU for the treatment of symptomatic chronic heart failure with reduced ejection fraction in adults with and without type-II diabetes. If approved for the broader indication in heart failure, Forxiga will be the first heart failure therapy indicated across the full ejection fraction range in Europe. In the United States, regulatory applications are under review for similar expanded use in HF.
In another press release AstraZeneca announced that a phase III study (PEARL), evaluating Imfinzi monotherapy in patients with Stage IV (metastatic) NSCLC whose tumor cells express high levels (25% or more) of PD-L1, did not achieve statistical significance for the primary endpoint of improving overall survival (OS). The study evaluated Imfinzi versus platinum-based chemotherapy. However, the study did see a clinically meaningful improvement in OS with Imfinzi monotherapy in the subset of patients with PD-L1 tumor expression greater than 50%, which was the study’s secondary endpoint.
Zacks Rank & Stocks to Consider
AstraZeneca has a Zacks Rank #3 (Hold). Some better-ranked large drugmakers are
Sanofi
SNY
and
Gilead Sciences
GILD
, both carrying a Zacks Rank #2 (Buy) at present. A small biotech worth considering is
Kamada
KMDA
, which sports a Zacks Rank #1 (Strong Buy). You can see
the complete list of today’s Zacks #1 Rank stocks here
.
Sanofi’s earnings per share estimates for 2022 have increased from $4.01 per share to $4.16 while that for 2023 has jumped from $4.22 per share to $4.31 in the past 60 days. Sanofi’s stock is down 6.2% in the year-to-date period.
Sanofi beat earnings expectations in all the trailing four quarters. The company delivered a four-quarter earnings surprise of 9.50%, on average.
Estimates for Gilead’s 2022 earnings per share have increased from $6.57 per share to $7.09, while that for 2023 have jumped from $6.48 per share to $6.78 in the past 60 days. Gilead’s stock is up 19.0% in the year-to-date period.
Gilead beat earnings expectations in three of the trailing four quarters. The company delivered a four-quarter earnings surprise of 0.36%, on average.
In the past 60 days, estimates for Kamada’s 2022 loss per share have narrowed from 14 cents to 7 cents. During the same period, the earnings estimates per share for 2023 have risen from 26 cents to 42 cents. Shares of Kamada have declined 36.5% in the year-to-date period.
Earnings of Kamada beat estimates in two of the last four quarters and missed the mark twice, witnessing a negative earnings surprise of 62.50%, on average.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








