AstraZeneca
AZN
and
Sanofi
SNY
published detailed data from their joint late-stage study MELODY, which is evaluating a single dose of their respiratory syncytial virus (RSV) vaccine candidate nirsevimab for protection of infants.
Data from the study demonstrated that the study met its primary endpoint of reducing the incidence of medically-attended lower respiratory tract infections (LRTI). A single dose of AstraZeneca and Sanofi’s nirsevimab reduced LRTI like bronchiolitis or pneumonia caused by RSV 74.5% compared to placebo in infants born at term or late preterm.
Another phase II/III study MEDLEY is evaluating nirsevimab in preterm infants and infants with congenital heart disease (CHD) and chronic lung disease (CLD) entering the first RSV season. Data from this study demonstrated that the vaccine candidate has a safety and tolerability profile similar to the only available preventative option for RSV, which is Synagis. Serum levels in the MEDLEY study were same as the levels achieved in the MELODY study. It implies similar protection for preterm infants, and infants with CHD and CLD compared to those born at term or late preterm.
Data from a pre-specified pooled analysis of phase III and phase II studies shows that nirsevimab achieved an efficacy of 77.3% in term and preterm infants against RSV-associated hospitalizations.
AstraZeneca and Sanofi are developing the vaccine candidate under a collaboration agreement. The European regulatory submission of AstraZeneca and Sanofi’s nirsevimab vaccine was accepted under accelerated assessment for RSV protection in all infants last month.
Shares of AstraZeneca and Sanofi have gained 38.7% and 14%, respectively, in the past year compared with the
industry
’s rise of 18.4%.
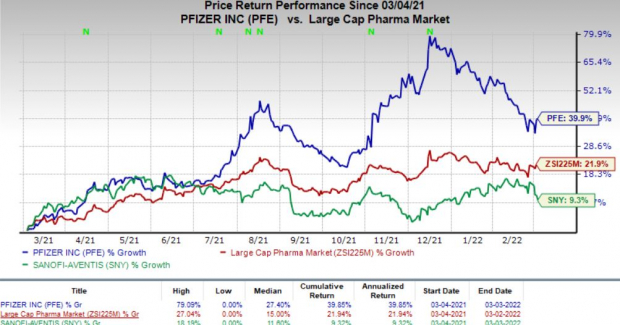
Image Source: Zacks Investment Research
RSV is a common and pervasive cause of acute respiratory illness, which usually starts in the fall months. This highly contagious virus affects the lungs and airways.
Some other companies are also developing their respective vaccines for treating RSV.
Earlier this week, the FDA granted a Breakthrough Therapy designation to
Pfizer
’s
PFE
RSV vaccine candidate RSVpreF. PFE is developing the vaccine candidate as an active immunization of pregnant women to prevent RSV-associated LRTI in infants as well as for immunizing older adults.
Pfizer is currently conducting multiple late-stage studies on RSVpreF.
British pharma giant
Glaxo
GSK
is evaluating its maternal RSV vaccine candidate in the phase III GRACE study. The study is evaluating the efficacy of a single dose of the unadjuvanted candidate in pregnant women.
Glaxo is also developing RSV vaccines for pediatric patients and older adults using different novel technologies.
Zacks Rank
AstraZeneca and Sanofi currently carry a Zacks Rank #3 (Hold) each. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2022?
From inception in 2012 through 2021, the
Zacks Top 10 Stocks
portfolios gained an impressive +1,001.2% versus the S&P 500’s +348.7%. Now our Director of Research has combed through 4,000 companies covered by the Zacks Rank and has handpicked the best 10 tickers to buy and hold. Don’t miss your chance to get in…because the sooner you do, the more upside you stand to grab.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









