From cell phones and laptops, to cars and grooming tools, lithium-ion batteries persist in almost every aspect of our life today. Perhaps with recent incidences of phones catching fire, researchers have found a potential successor to the flammable lithium-ion batteries.
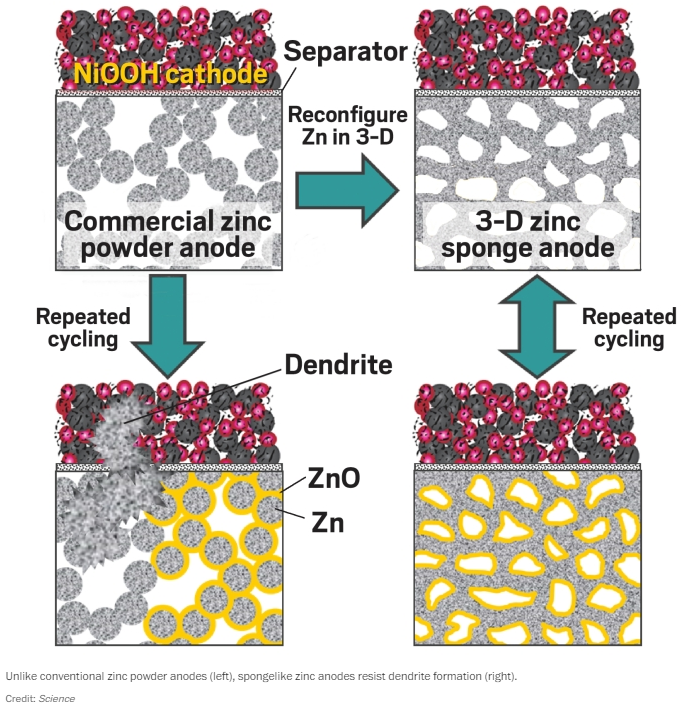
Traditionally, aqueous zinc based batteries tend to fail quickly. This is because cycles of recharging cause zinc atoms to pile up, forming dendrites (tiny zinc branches) that are capable of piercing, and short circuiting the battery. According to a study however, these issues can be mitigated by forming zinc electrodes into sponge like structures.
This new research places Zinc advantageously when competing with other battery potentials, as zinc is more abundant than lithium, making it a cheaper alternative. Additionally, your phones won’t risk combustion, as Zinc batteries are significantly safer due to the use of aqueous electrolytes rather than flammable organics found in lithium-ion batteries.
Through a mixture of oil, water, and zinc powder, the team — consisted of Joseph F. Parker, Jeffrey W. Long, and Debra R. Rolison — created the sponge like structure by letting it settle overnight.
Thanks to its new sponge like structure, a more uniform oxidation of zinc, and ultimately more even coating of zinc oxide (the discharge product) on the sponge anode is achieved. As such, a more uniform reverse reaction of zinc oxide back to the metallic zinc is also achieved, evidencing further stability.
Joseph F. Parker notes that even once the zinc metal is 90% oxidized during discharge, the sponge stays resilient, maintaining its metallic zinc core. He adds that the core makes it physically difficult for dendrites to form due to the uniform distribution of electric currents throughout the sponge.
In testing, the team of researchers found that the sponge structure of zinc electrodes protected a Ni-Zn battery against dendrite formation. The battery continued to impress by withstanding tens of thousands of cycles with micro hybrid vehicles.
In the words of Paul V. Braun, a professor of materials science and chemistry at the University of Illinois, “For quite some time, this team and others have been attempting to use 3-D structured electrodes to enhance rechargeable battery performance”.
Braun continues, commending that the NRL team “has found a particularly compelling system, where the 3-D electrode structure provides high power, as expected, but perhaps surprisingly, results in dendrite suppression and thus very good long term cycling… this discovery is particularly useful because it is accomplished with an intrinsically safe, earth abundant, and relatively high-energy-density nickel-zinc chemistry.”
Featured Image: depositphotos/garloon










