The biotech sector has been in the spotlight in the past week with important pipeline and regulatory updates before the second-quarter earnings kickstart.
Recap of the Week’s Most Important Stories
:
Incyte’s Drug Gets FDA Nod for Another Indication
:
Incyte
INCY
announced that the FDA has
approved
Opzelura (ruxolitinib) cream for another indication. The regulatory body approved Opzelura cream 1.5% for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older. The FDA approval was based on data from the phase III TRuE-V clinical trial program (TRuE-V1 and TRuE-V2), which evaluated the safety and efficacy of Opzelura versus vehicle in more than 600 people with nonsegmental vitiligo, aged 12 and older. Opzelura was earlier approved by the FDA for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis (AD) in non-immunocompromised patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
Incyte currently carries a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Apellis Up on Regulatory Update
:
Apellis Pharmaceuticals, Inc
.
APLS
surged after it announced that the FDA has accepted and granted Priority Review designation for the new drug application (NDA) for pipeline candidate pegcetacoplan. The NDA is seeking approval of pegcetacoplan, an investigational, targeted C3 therapy, for treating geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The regulatory body has set a target action date of Nov 26, 2022. The FDA has stated that it is not currently planning to hold an advisory committee meeting to discuss the application. The NDA was submitted based on results from the phase III DERBY and OAKS studies at 12 and 18 months and the phase II FILLY study at 12 months. Apellis plans to submit a marketing authorization application to the European Medicines Agency in the second half of 2022.
Novavax Surges on CDC Endorsement
:
Novavax
NVAX
shares gained after the company announced that the Centers for Disease Control and Prevention (“CDC”) has endorsed its protein-based COVID-19 vaccine called Novavax COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) as a primary two-dose regimen for use in adults aged 18 years and older. The endorsement follows Advisory Committee on Immunization Practices’ (“ACIP”) recommendation of using Novavax’s vaccine in adults. The CDC establishes its vaccine recommendations and schedules based on advice from ACIP. Last week, Novavax was granted emergency use authorization (EUA) for its COVID-19 vaccine as a primary two-dose regimen for use in adults.
NexImmune Application Gets Clearance
:
NexImmune
NEXI
announced that the FDA has given clearance to its investigational new drug (IND) application for NEXI-003. Following the IND clearance, NexImmune can initiate a study to evaluate NEXI-003 in patients with relapsed or refractory HPV-related cancers. Shares of this clinical-stage biotechnology company jumped 23.13% in aftermarket hours trading on Jul 14 after this announcement. NEXI-003, an autologous antigen-specific T cell product (CD3+/CD4-), is being developed for patients with relapsed or refractory human papillomavirus (HPV)-related cancers.
The phase I study will enroll patients at multiple clinical sites across the United States. The proposed study is a two-part, multicenter, open-label, dose-finding, first-in-human (FIH) study to characterize the safety and clinical activity of NEXI-003 in patients with relapsed or refractory locally advanced or metastatic HPV-related oropharyngeal cancers (with confirmed histopathology detection of HPV-16 and/or HPV-18 expression), who have received at least one prior regimen of standard therapy according to local standard of care guidance(s).
Performance
The Nasdaq Biotechnology Index has gained 0.57% in the past five trading sessions. Among the biotech giants, Incyte has gained 1.17% during the period. Over the past six months, shares of Vertex have surged 25.45%. (See the last biotech stock roundup here:
Biotech Stock Roundup: ICPTs Study Data, NVAX Vaccine Gets EUA, VERU Up on Data
)
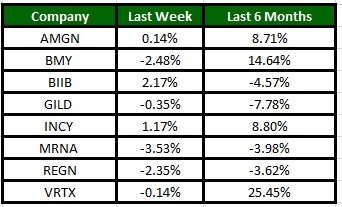
Image Source: Zacks Investment Research
What’s Next in Biotech?
Stay tuned for earnings and other updates.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +24.8% per year. So be sure to give these hand-picked 7 your immediate attention.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








