The biotech sector has been in the spotlight in the past week, with regular pipeline and regulatory updates.
Recap of the Week’s Most Important Stories
:
Updates From Bristol Myers
:
Bristol Myers Squibb
BMY
announced that the FDA has
approved
Opdivo (nivolumab) combinations for yet another indication. The regulatory body approved the drug (injection for intravenous use) in combination with fluoropyrimidine- and platinum-containing chemotherapy as a first-line treatment of adult patients with unresectable advanced or metastatic esophageal squamous cell carcinoma (ESCC) regardless of PD-L1 status. Concurrently, Opdivo in combination with Yervoy (ipilimumab) was also approved for the same indication by the FDA.
The approvals are based on the phase III CheckMate -648 trial, which evaluated Opdivo in combination with chemotherapy and Opdivo plus Yervoy, each compared to chemotherapy alone. Data showed that Opdivo in combination with chemotherapy demonstrated superior overall survival (OS) compared to chemotherapy alone, both in all randomized patients.
Bristol Myers also announced results from a multicenter, phase II study through the primary analysis of the chimeric antigen receptor (CAR) T cell therapy Breyanzi (lisocabtagene maraleucel) in adults with refractory or relapsed large B-cell lymphoma (LBCL) after first-line therapy. Initial results showed Breyanzi delivered complete responses in more than half of the patients — who were not deemed fit for stem cell transplant — with refractory or relapsed large B-cell lymphoma after the first-line therapy.
Pipeline Updates From Biogen
:
Biogen
BIIB
and partner Denali Therapeutics announced that dosing
started
in the global phase IIb LUMA study to evaluate the efficacy and safety of BIIB122 (DNL151) compared to placebo in approximately 640 participants with early-stage Parkinson’s disease. BIIB122 is an inhibitor of LRRK2, a potential novel target intended to impact the underlying biology and slow the progression of Parkinson’s disease. It was discovered and developed by Denali. The phase IIb multi-center, randomized, double-blind, placebo-controlled study will evaluate the safety and efficacy of BIIB122 in people with early-stage Parkinson’s disease, aged between 30 years and 80 years.
Separately, Biogen and partner
Sage Therapeutics, Inc
.
SAGE
announced positive results from the late-stage SKYLARK study of the pipeline candidate zuranolone, an investigational two-week, once-daily oral drug, which is being evaluated in women with postpartum depression (PPD). The study met its primary and all key secondary endpoints. Zuranolone 50 mg demonstrated a statistically significant and clinically meaningful improvement in depressive symptoms at Day 15, denoting the primary endpoint, and at days 3, 28, and 45, suggesting the key secondary endpoints. Zuranolone is also being developed for major depressive disorder (MDD).
Sage Therapeutics and Biogen initiated a rolling submission of a new drug application (NDA) to the FDA for zuranolone to treat MDD and also plan to complete the MDD NDA filing in the second half of 2022. An associated NDA filing for PPD is anticipated in early 2023.
Regulatory Updates From Regeneron
:
Regeneron (
REGN
)
and partner
Sanofi
SNY
announced that the FDA
accepted
for Priority Review the supplemental biologics license application (sBLA) for asthma drug Dupixent (dupilumab). The sBLA is seeking label expansion of the drug for treating adults with prurigo nodularis, a chronic skin disease that causes extreme itch and inflammatory skin lesions (nodules). The target action date set by the regulatory body is Sep 30, 2022.
Dupixent is being jointly marketed by Regeneron and Sanofi under a global collaboration agreement. Sanofi records global net product sales of Dupixent while Regeneron records its share of profits/losses in connection with the global sales of the drug. Dupixent is approved for use in certain patients with atopic dermatitis, asthma, CRSwNP or eosinophilic esophagitis in different age populations in a number of countries around the world.
Regeneron also announced that it completed the previously announced acquisition of Checkmate Pharmaceuticals, Inc. Checkmate’s lead investigational candidate vidutolimod is an advanced generation CpG-A oligodeoxynucleotide Toll-like receptor 9 (TLR9) agonist delivered in a virus-like particle (VLP) and also demonstrated clinical responses as a monotherapy in patients with PD-1 refractory melanoma.
Regeneron currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Performance
The Nasdaq Biotechnology Index has gained 1.54% in the past four trading sessions. Among the biotech giants, Moderna has gained 10.05% during the period. Over the past six months, shares of Moderna have lost 58.76%. (See the last biotech stock roundup here:
Biotech Stock Roundup: ETTX Up on Acquisition Deal, REGN Offers Updates
)
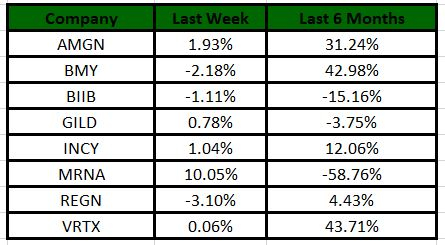
Image Source: Zacks Investment Research
What’s Next in Biotech?
Stay tuned for more pipeline and regulatory updates.
How to Profit from the Hot Electric Vehicle Industry
Global electric car sales in 2021 more than doubled their 2020 numbers. And today, the electric vehicle (EV) technology and very nature of the business is changing quickly. The next push for future technologies is happening now and investors who get in early could see exceptional profits.
See Zacks’ Top Stocks to Profit from the EV Revolution >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









