The biotech sector has been in the news in the past week due to key regulatory and pipeline updates.
Nektar Therapeutics
NKTR
plummeted on news of study failure of its lead drug while
Moderna
MRNA
announced pipeline progress.
Recap of the Week’s Most Important Stories
:
Nektar Plunges on Drug Failure
: Shares of Nektar
plunged
significantly after the company and partner
Bristol Myers Squibb
BMY
announced disappointing results from the phase III study — PIVOT IO-001. The study was evaluating the doublet therapy of Nektar’s lead candidate bempegaldesleukin in combination with Bristol Myers’ Opdivo (nivolumab) compared to Opdivo monotherapy as a first-line treatment for previously untreated unresectable or metastatic melanoma. The study did not meet the primary endpoints of progression-free survival (PFS) and objective response rate (ORR) as assessed by Blinded Independent Central Review on the basis of the review of the study for efficacy and safety by an independent Data Monitoring Committee (“DMC”). The DMC also notified both the companies that the third primary endpoint of overall survival (OS) did not meet statistical significance at the first interim analysis.
Consequently, Nektar and Bristol-Myers have decided to unblind the study and not perform additional analyses for the OS endpoint. Moreover, based on the results from PIVOT IO-001, the companies have decided to discontinue enrollment and unblind the ongoing PIVOT-12 study in adjuvant melanoma, which is evaluating the doublet therapy of bempegaldesleukin in combination with Opdivo compared to Opdivo monotherapy in patients at high risk for recurrence after complete resection of melanoma.
Updates From Moderna
: Moderna announced that the first participant has been
dosed
in a phase I study of its experimental human immunodeficiency virus (HIV) trimer mRNA vaccine (mRNA-1574). The open-label, multicenter, randomized phase I study (HVTN 302) is designed to evaluate the safety and immunogenicity of experimental HIV trimer mRNA vaccines. The study is expected to enroll approximately 100 HIV-negative adults aged 18 to 55 years. The study is sponsored and funded by the Division of AIDS of the National Institute of Allergy and Infectious Diseases within the National Institutes of Health.
Earlier, Moderna announced that the first participant has been dosed in the mid-stage study of its Omicron-specific bivalent booster candidate (mRNA-1273.214). This bivalent booster candidate (mRNA-1273.214) combines Moderna’s Omicron-specific booster candidate (mRNA-1273.529) and its COVID-19 vaccine (mRNA-1273), which was developed to protect against the original COVID-19 strain. The study will evaluate the immunogenicity, safety and reactogenicity of the candidate as a single booster dose in adults aged 18 years and above. These adults must have previously received the two-dose primary series of Moderna’s original COVID-19 vaccine and a 50 µg booster dose of mRNA-1273, with the booster dose being administered at least three months ago.
Exelixis Announces Liver Cancer Study
Results
:
Exelixis
EXEL
announced results from the final
analysis
of the second primary endpoint of OS from the late-stage COSMIC-312 study, which evaluated its lead drug Cabometyx (cabozantinib) in combination with Tecentriq versus Nexavar in patients with previously untreated advanced hepatocellular carcinoma (HCC). Patients were randomized approximately 2:1:1 to one of three arms: Cabometyx (40 mg) in combination with Tecentriq (n=432), Nexavar (n=217) or Cabometyx (60 mg; n=188). The results showed that the combination of Cabometyx, Tecentriq or Nevxavar did not improve OS nor worsened the same. Consequently, based on this outcome for OS and the rapidly evolving treatment landscape for previously untreated advanced HCC, Exelixis does not intend to submit a supplemental new drug application to the FDA.
EXEL currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Regulatory Update From Incyte
:
Incyte
INCY
announced
that the FDA has extended the review period for the supplemental New Drug Application (sNDA) for ruxolitinib cream, which was seeking approval of the cream for the treatment of vitiligo. The regulatory body has extended the target action date by three months to Jul 18, 2022. The sNDA was originally accepted and granted priority review by the FDA in December 2021 and granted a target action date of Apr 18, 2022. The FDA extended the target action date to review additional data from the ongoing phase III studies submitted by Incyte in response to the FDA’s information request. The FDA has determined the submission of the additional information to constitute a major amendment to the sNDA, resulting in an extension of the target action date.
Performance
The Nasdaq Biotechnology Index has gained 0.55% in the past five trading sessions. Among the biotech giants, Moderna has gained 14.57% during the period. Over the past six months, shares of Moderna have lost 65.91%. (See the last biotech stock roundup here:
Biotech Stock Roundup: OCGN Down on Update, VYGR Surges on Deal & Other Updates
)
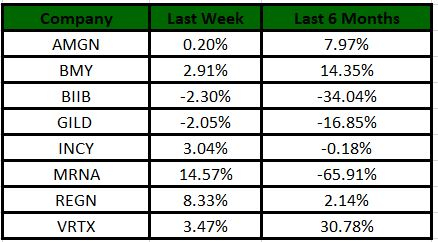
Image Source: Zacks Investment Research
What’s Next in Biotech?
Stay tuned for more pipeline and regulatory updates.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









