bluebird bio
BLUE
announced that the FDA lifted its partial clinical hold for patients under the age of 18 in studies evaluating lovotibeglogene autotemcel (lovo-cel) for sickle cell disease (SCD).
bluebird plans to resume enrollment and treatment of pediatric and adolescent patients in the first quarter of next year.
Lovotibeglogene autotemcel gene therapy is an investigational one-time treatment being studied for SCD, that is designed to add functional copies of a modified form of the β-globin gene (βA-T87Q-globin gene) into a patient’s own hematopoietic (blood) stem cells (HSCs).
The studies were put on partial hold for these patients in December 2021 following an investigation by bluebird bio into an adolescent patient with persistent, non-transfusion-dependent anemia following treatment with lovo-cel.
bluebird recently detailed its investigation of this case at the American Society of Hematology (ASH) Annual Meeting and Exposition alongside details from another case of persistent anemia in an adult patient following treatment with lovo-cel.
The company stated that both patients had two α-globin gene deletions (−α3.7/−α3.7), also known as alpha-thalassemia trait and notably were the only patients in the study with this specific genotype. Following these cases, this genotype was added to the exclusion criteria for ongoing studies.
Meanwhile, enrollment and dosing for adult patients 18 and older in the HGB-210 study continued as planned, while the partial hold was ongoing for patients under the age of 18. bluebird is working to resume enrollment and treatment of patients ages 2-17 consistent with the study protocol and intends to submit a biologics license application (BLA) to the FDA for lovo-cel in the first quarter of 2023.
bluebird has completed treatment of all patients in HGB-206 Group C, which will form the primary basis for efficacy in its lovo-cel BLA submission and expects to complete vector and drug product analytical comparability studies for the same shortly.
The FDA had previously granted orphan drug designation, fast track designation, regenerative medicine advanced therapy (RMAT) designation and rare pediatric disease designation for lovo-cel.
Shares of bluebird have declined 28.2% in the year-to-date period compared with the
industry
’s fall of 21.2%.
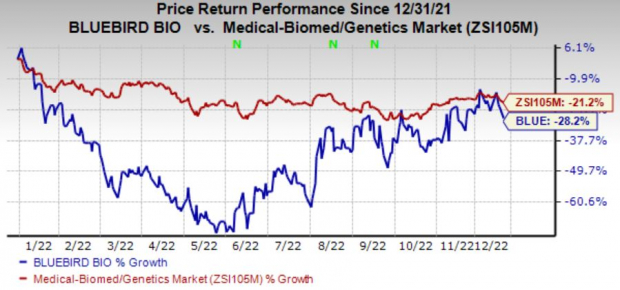
Image Source: Zacks Investment Research
It has been an eventful year for the company. bluebird received approvals for two of its gene therapies, Zynteglo (beti-cel) for transfusion-dependent thalassemia (TDT) and Syskona (eli-cel) for active cerebral adrenoleukodystrophy (CALD) in 2022.
Zynteglo was granted FDA approval in August as a one-time gene therapy customized for treating adults and pediatric patients with beta-thalassemia requiring regular red blood cell (RBC) transfusions across all genotypes.
A potential approval of lovo cell will further boost the growth prospects of the company.
While the therapies promise potential, gene therapies are complex by nature. Thus, Zynteglo and Syskona are pretty complex to administer and can only be provided as treatments to patients by qualified treatment centers, apart from being expensive treatments.
Hence, it remains to be seen how these therapies fare in the market.
Zacks Rank and Key Picks
bluebird currently has a Zacks Rank #3 (Hold). Some better-ranked stocks in the overall healthcare sector are
Gilead Sciences, Inc
.
GILD
,
Kala Pharmaceuticals
KALA
and
Immunocore
IMCR
. All three companies carry a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Earnings estimates for Gilead Sciences have increased 48 cents in the last 60 days to $7.09. Gilead has surpassed earnings estimates in three of the past four quarters with an average beat of 0.36%.
Earnings estimates for Kala Pharmaceuticals have increased 26 cents in the last 30 days to $13.32. Kala has surpassed earnings estimates in two of the past four quarters with an average beat of 2.39%.
Loss estimates for Immunocore have narrowed 34 cents in the last 30 days to 54 cents. Immunocore has surpassed earnings estimates in three of the past four quarters with an average beat of 68.34%.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









