PR Newswire
– CHMP recommendation for approval of NULIBRY in the European Union (EU) for the treatment of patients with molybdenum cofactor deficiency (MoCD) Type A is based on the efficacy and safety data collected to date compared to data from a natural history study
– Under an accelerated assessment pathway, a decision by the European Commission (EC), which authorizes marketing approval in the European Union (EU), is expected on the NULIBRY application later this year
–
If approved by the EC, NULIBRY would be the first and only approved therapy in the EU to treat patients with MoCD type A, an ultra-rare, life-threatening genetic disorder that often progresses rapidly in infants with a median overall survival age of about four years
– NULIBRY was BridgeBio’s first FDA-approved therapeutic; Sentynl acquired global rights to NULIBRY in
March 2022
PALO ALTO, Calif.
and
SOLANA BEACH, Calif.
,
July 25, 2022
/PRNewswire/ — BridgeBio Pharma, Inc. (Nasdaq: BBIO) (BridgeBio), a commercial-stage biopharmaceutical company that focuses on genetic diseases and cancers, and Sentynl Therapeutics, Inc. (Sentynl), a U.S.-based biopharmaceutical company focused on bringing innovative therapies to patients living with rare diseases owned by Zydus Lifesciences Ltd. (formerly known as Cadila Healthcare Ltd.), today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended that the European Commission authorize marketing under exceptional circumstances for NULIBRY
®
(fosdenopterin) for Injection as the first therapy for the treatment of patients with molybdenum cofactor deficiency (MoCD) Type A. MoCD Type A is an ultra-rare and progressive condition, known to impact less than 150 patients globally with a median survival of four years.
NULIBRY is a first-in-class cPMP substrate replacement therapy that was approved by the U.S. Food and Drug Administration (FDA) in 2021 to reduce the risk of mortality in patients with MoCD Type A. If approved by the European Commission, NULIBRY would be the first and only approved therapy in the EU for MoCD Type A.
In
March 2022
, Sentynl acquired the global rights to NULIBRY and is responsible for the ongoing development and commercialization of NULIBRY in
the United States
and developing, manufacturing, and commercializing fosdenopterin globally. Sentynl and BridgeBio share development responsibilities through the approval of the marketing authorization application under accelerated assessment with the EMA and through approval of NULIBRY’s regulatory submission with the Israeli Ministry of Health.
“Our work on NULIBRY and MoCD Type A epitomizes BridgeBio’s belief that no disease is too rare to address. With this positive CHMP opinion, we are closer to delivering a treatment option to all children across the globe who suffer with MoCD Type A,” said BridgeBio founder and CEO
Neil Kumar
, Ph.D.
The positive CHMP opinion is supported by data from three clinical trials that demonstrated efficacy of NULIBRY for the treatment of patients with MoCD Type A compared to data from a natural history study. These studies showed that NULIBRY reduced the risk of death by 86% and increased the probability of survival to 86% at three years compared to 52% in the untreated, genotype-matched, historical control group in the natural history study.
“We are thrilled by the CHMP’s recommendation in favor of NULIBRY and hope that patients living with MoCD Type A in
Europe
and around the world can access this therapy,” said
Matt Heck
, CEO of Sentynl. “The CHMP positive opinion marks important progress not only for the program but also for the MoCD Type A patients outside of the U.S. who are seeking ways to treat their life-threatening and progressive disease.”
Based on the CHMP recommendation, a decision by the EC, which authorizes marketing applications in the EU, is expected on the NULIBRY application later this year. The recommendation for marketing authorization under exceptional circumstances is granted to medicines where the applicant is unable to provide comprehensive data under normal conditions of use because the disease being treated is so rare.
In
April 2022
, BridgeBio received New Drug Application (NDA) Approval in Principle from the Israeli Ministry of Health and the application is currently undergoing the final review processes.
About Molybdenum Cofactor Deficiency (MoCD) Type A
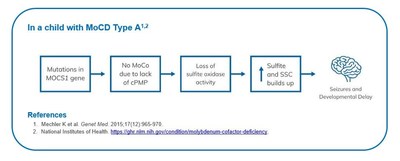
MoCD Type A is an autosomal recessive, inborn error of metabolism caused by mutations in the molybdenum cofactor synthesis 1 gene and characterized by a deficiency in molybdenum cofactor production, leading to a lack of molybdenum-dependent enzyme activity.
1,2
The lack of activity leads to decreased sulfite oxidase activity with buildup of sulfite and secondary metabolites (such as S-sulfocysteine) in the brain, which causes irreversible neurological damage.
2
MoCD Type A is an ultra-rare disease. The incidence and prevalence of MoCD Type A in the European Union are not known, but the estimated prevalence is 0.005 per 10,000 persons. Based on these estimates, MoCD Type A is likely to be underdiagnosed.
The most common presenting symptoms of MoCD Type A are seizures, feeding difficulties and encephalopathy. Patients with MoCD Type A who survive beyond infancy typically suffer from progressive brain damage, which presents in characteristic patterns on magnetic resonance imaging (MRI). This damage leads to severe psychomotor impairment and an inability to make coordinated movements or communicate with their environment.
About NULIBRY
®
(Fosdenopterin) for Injection
NULIBRY®(Fosdenopterin) for Injection is a substrate replacement therapy that provides an exogenous source of cPMP, which is converted to molybdopterin. Molybdopterin is then converted to molybdenum cofactor, which is needed for the activation of molybdenum-dependent enzymes, including sulfite oxidase, an enzyme that reduces levels of neurotoxic sulfites. It is the first and only FDA-approved therapy indicated to reduce the risk of mortality in patients with MoCD Type A, and clinical trials have demonstrated that patients treated with NULIBRY or rcPMP had an improvement in overall survival compared to the untreated, genotype-matched, historical control group.
References
1
Mechler K et al. Genet Med. 2015;17(12):965-970.
2
Schwarz G. Cur Op in
Che Bio
. 2016;31:179-187.
About BridgeBio Pharma, Inc.
BridgeBio Pharma, Inc. (BridgeBio) is a commercial-stage biopharmaceutical company founded to discover, create, test and deliver transformative medicines to treat patients who suffer from genetic diseases and cancers with clear genetic drivers. BridgeBio’s pipeline of development programs ranges from early science to advanced clinical trials. BridgeBio was founded in 2015 and its team of experienced drug discoverers, developers and innovators are committed to applying advances in genetic medicine to help patients as quickly as possible. For more information visit
bridgebio.com
and follow us on
LinkedIn
and
Twitter
.
About Sentynl Therapeutics
Sentynl Therapeutics is a U.S.-based biopharmaceutical company focused on bringing innovative therapies to patients living with rare diseases. The company was acquired by the Zydus Group in 2017. Sentynl’s experienced management team has previously built multiple successful pharmaceutical companies. With a focus on commercialization, Sentynl looks to source effective and highly differentiated products across a broad spectrum of therapeutic areas to address unmet needs. Sentynl is committed to the highest ethical standards and compliance with all applicable laws, regulations, and industry guidelines. For more information, visit
www.sentynl.com
.
About Zydus
The Zydus Group, with an overarching purpose of empowering people with freedom to live healthier and more fulfilled lives, is an innovative, global pharmaceutical company that discovers, develops, manufactures, and markets a broad range of healthcare therapies. The group employs over 23000 people worldwide and is driven by its mission to unlock new possibilities in life-sciences through quality healthcare solutions that impact lives. The group aspires to become a global life-sciences company transforming lives through pathbreaking discoveries. For more information, visit
https://www.zyduslife.com/zyduslife
/.
BridgeBio Pharma, Inc. Forward-Looking Statements
This press release contains forward-looking statements. Statements we make in this press release may include statements that are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are usually identified by the use of words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “seeks,” “should,” “will,” and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward-looking statements, including statements relating to, the timing and outcome of the EC’s decision on NULIBRY, the potential ability for NULIBRY to be the first and only approved therapy in the EU to treat patients with MoCD type A, and the potential ability to provide treatment options to MoCD Type A patients in
Europe
and around the world, reflect our current views about our plans, intentions, expectations, strategies and prospects, and are based on the information currently available to us and on assumptions we have made and are not forecasts, promises nor guarantees. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by these forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a number of risks, uncertainties and assumptions, including, but not limited to, the success of our ongoing collaboration with Sentynl, including our co-development responsibilities through approval of the marketing authorization application under accelerated assessment with the EMA and through approval of NULIBRY’s regulatory submission with the Israeli Ministry of Health, Sentynl’s ability to successfully develop and commercialize NULIBRY in
the United States
and to develop, manufacture, and commercialize fosdenopterin globally, , as well as those risks set forth in the Risk Factors section of our most recent Annual Report on Form 10-K and BridgeBio Pharma’s other SEC filings. Moreover, we operate in a very competitive and rapidly changing environment in which new risks emerge from time to time. Except as required by applicable law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
BridgeBio Contact:
Grace Rauh
[email protected]
(917) 232-5478
Sentynl Therapeutics Contact:
Michael Hercz
[email protected]
(888) 507-5296
![]()
View original content to download multimedia:
https://www.prnewswire.com/news-releases/bridgebio-pharma-and-sentynl-therapeutics-receive-positive-chmp-opinion-for-nulibry-fosdenopterin-for-the-treatment-of-mocd-type-a-301592171.html
SOURCE Sentynl Therapeutics