Bristol Myers
BMY
announced that the European Commission (EC) approved a label expansion of its blockbuster drug Opdivo (nivolumab)-based combinations for several indications.
The drug has been approved in combination with fluoropyrimidine- and platinum-based chemotherapy for the first-line treatment of adult patients with unresectable advanced, recurrent or metastatic esophageal squamous cell carcinoma (ESCC) with tumor cell PD-L1 expression ≥ 1%.
The EC’s decision is based on the phase III CheckMate -648 study wherein Opdivo with chemotherapy demonstrated a statistically significant and clinically significant overall survival (OS) benefit compared with chemotherapy alone at the pre-specified interim analysis.
The regulatory body also approved Opdivo in combination with Yervoy (ipilimumab) for the first-line treatment of adult patients with unresectable advanced, recurrent or metastatic ESCC with tumor cell PD-L1 expression ≥ 1%.
The EC also approved Opdivo for the adjuvant treatment of adults with muscle-invasive urothelial carcinoma with tumor cell PD-L1 expression ≥1% who are at a high risk of recurrence after undergoing radical resection.
This approval was based on the phase III CheckMate -274 study results, which showed that adjuvant treatment with Opdivo significantly reduced patients’ risk of disease recurrence or death compared with placebo.
Per the company, Opdivo is now the first and only adjuvant immunotherapy option approved in this setting in the European Union.
Consequently, Opdivo has now been approved for the adjuvant treatment of three different carcinoma types in the EU — urothelial carcinoma, melanoma and esophageal/gastroesophageal junction cancer. In addition, BMY has a broad development program in earlier stages of cancer that currently spans eight different tumor types across the neoadjuvant, adjuvant and peri-operative settings.
The performance of immuno-oncology drug Opdivo, approved for multiple cancer indications, revived after a slowdown early in 2021. Opdivo is one of the top revenue generators for BMY and the continued label expansion of the drug for additional indications should further boost its growth potential.
Shares of Bristol Myers have rallied 20.1% year to date against the
industry
’s decline of 10.9%.
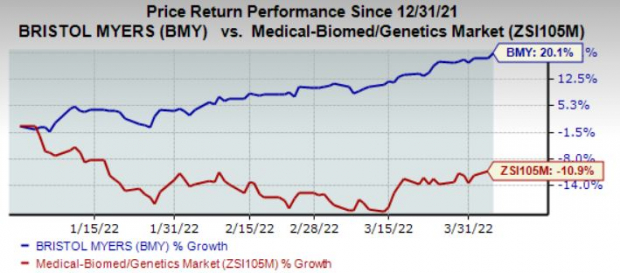
Image Source: Zacks Investment Research
The FDA recently approved a new, first-in-class, fixed-dose combination of Opdivo and relatlimab (novel LAG-3-blocking antibody) administered as a single intravenous infusion for the treatment of adult and pediatric patients 12 years of age or older with unresectable or metastatic melanoma (a kind of skin cancer). The drug is approved under the brand name Opdualag.
The approval of these new drugs adds a revenue stream, which should boost growth in the coming quarters.
In 2021, Bristol Myers obtained 20 approvals for new drugs and additional indications and formulations of currently marketed drugs in major markets, including regulatory approvals of Breyanzi and Abecma in hematology malignancies. The FDA approval of Zeposia for the treatment of adults with moderately to severely active UC has expanded the company’s portfolio in immunology. Bristol Myers is also developing deucravacitinib, its TYK2 inhibitor, for the treatment of psoriasis and other immune-mediated diseases.
BMY expanded its leading cardiovascular franchise with the addition of mavacamten from the acquisition of MyoKardia in 2020.
However, competition is also stiff for Opdivo from the likes of
Merck
’s
MRK
Keytruda. The drug, approved for various oncology indications, is the key driver for MRK.
Additional label expansion of Keytruda will boost Merck’s top line.
Bristol-Myers currently carries a Zacks Rank #3 (Hold). A few better-ranked stocks in the biotech sector are
Voyager Therapeutics
VYGR
and
VistaGen Therapeutics
VTGN
, both carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Loss estimates for VYGR have narrowed to $1.35 from $2.20 for 2022 in the past 60 days. Earnings of Voyager surpassed estimates in three of the trailing four quarters and missed the same once.
Loss estimates for VTGN have narrowed to 22 cents from 27 cents for 2022 in the past 60 days. Earnings of VistaGen surpassed estimates in two of the trailing four quarters and missed the same twice.
Just Released: The Biggest Tech IPOs of 2022
For a limited time, Zacks is revealing the most anticipated tech IPOs expected to launch this year. Concerns about Federal interest rates and inflation caused many private companies to stay on the bench- leading to companies with better brand recognition and higher growth rates getting into the game. With the strength of our economy and record amounts of cash flooding into IPOs, you don’t want to miss this opportunity. See the complete list today.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








