Bristol Myers Squibb
BMY
announced that the FDA has approved Opdivo (nivolumab) combinations for yet another indication.
The regulatory body approved the drug (injection for intravenous use) in combination with fluoropyrimidine- and platinum-containing chemotherapy as a first-line treatment of adult patients with unresectable advanced or metastatic esophageal squamous cell carcinoma (ESCC) regardless of PD-L1 status.
Concurrently, Opdivo in combination with Yervoy (ipilimumab) was also approved for the same indication by the FDA.
The approvals are based on the phase III CheckMate -648 trial, which evaluated Opdivo in combination with chemotherapy and Opdivo plus Yervoy, each compared to chemotherapy alone. Data showed that Opdivo in combination with chemotherapy demonstrated superior overall survival (OS) compared to chemotherapy alone, both in all randomized patients.
Opdivo plus Yervoy also improved OS compared to chemotherapy in all-randomized patients, indicating the secondary endpoint while patients whose tumors express PD-L1 (≥1%), denoted the primary endpoint.
Opdivo either alone or in combination with chemotherapy or Yervoy is already approved for various indications. Opdivo plus Yervoy is approved for the treatment of adult patients with unresectable or metastatic melanoma, metastatic non-small cell lung cancer (NSCLC) and advanced renal cell carcinoma (RCC) among others.
Opdivo is one of the top revenue generators for BMY and the continued label expansion of the drug for additional indications should further boost growth. In particular, demand for the drug to treat first-line lung, renal and gastric cancer as well as adjuvant esophageal and bladder cancers boosted its growth.
Opdivo and Yervoy continued their growth trajectory in the first quarter of 2022 and recorded a double-digit rise on continued demand for its newly-launched indications.
Shares of Bristol Myers have rallied 22.1% year to date against the
industry
’s decline of 22%.
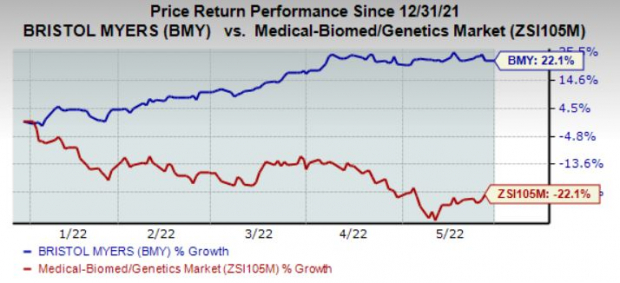
Image Source: Zacks Investment Research
The FDA earlier approved a new, first-in-class, fixed-dose combination of Opdivo and relatlimab (novel LAG-3-blocking antibody), administered as a single intravenous infusion to treat adult and pediatric patients, aged 12 years and above with unresectable or metastatic melanoma (a kind of skin cancer).
The drug is approved under the brand name Opdualag.
Approval of these new drugs adds a revenue stream, which should bolster growth in the coming quarters and counter the adverse impact of generic competition for the lead drug Revlimid.
The FDA also approved cardiovascular drug Camzyos (mavacamten, 2.5 mg, 5 mg, 10 mg, 15 mg capsules) for the treatment of adults with symptomatic New York Heart Association (NYHA) class II-III obstructive hypertrophic cardiomyopathy (obstructive HCM) to improve functional capacity and symptoms.
However, competition is stiff for Opdivo from the likes of
Merck
’s
MRK
blockbuster drug Keytruda. Approved for various oncology indications, Keytruda is MRK’s key driver.
Continued label expansions of the drug drove Merck’s top line.
Bristol Myers currently carries a Zacks Rank #3 (Hold). Two better-ranked stocks are
Alkermes
ALKS
and
Geron Corporation
GERN
. While Alkermes sports a Zacks Rank #1 (Strong Buy), Geron has a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank stocks here
.
ALKS’ loss estimates for 2022 have narrowed to 3 cents from a loss of 14 cents in the past 60 days. Alkermes surpassed on earnings in all the trailing four quarters, the average being 350.48%.
GERN’s loss estimates for 2022 have narrowed 6 cents in the past 60 days. Geron surpassed on earnings in three of the trailing four quarters and missed the mark in the remaining one, the average surprise being 1.07%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








