Clovis Oncology
CLVS
reported first-quarter 2022 net loss of 44 cents per share, narrower than the year-ago period’s loss of 64 cents. However, the bottom line was wider than the Zacks Consensus Estimate of a loss of 43 cents per share.
Net revenues — entirely from Clovis’ only marketed drug PARP inhibitor Rubraca — were down 10% year over year to $34.2 million, missing the Zacks Consensus Estimate of $37.0 million.
Shares of Clovis fell 9.1% on May 4 following the dismal earnings results. The stock has declined 22.5% in the year so far compared with the
industry
’s 21.2% decrease.
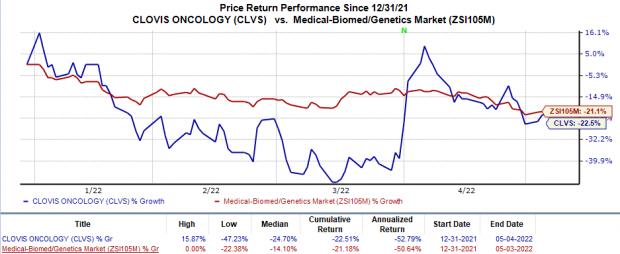
Image Source: Zacks Investment Research
Quarter in Detail
Sales of Rubraca in the United States were $24.5 million, down 22.7% year over year. Ex-U.S. market sales were $9.7 million for the first quarter, up 51.6% year over year. Lower sales were due to COVID-19 impacts as fewer patients were treated for ovarian cancer amid the pandemic.
For the first quarter, research & development expenses decreased 20% year over year to $42.3 million, primarily owing to lower spending on Rubraca clinical studies.
Selling, general and administrative expenses declined 2% year over year to $29.2 million.
Clovis ended the quarter with $122.2 million of cash equivalents and available-for-sale securities compared with $143.4 million on Dec 31, 2021.
CLVS expects to raise additional capital to support its operations for at least next 12 months and ahead.
Pipeline Updates
Label Expansion Studies on Rubraca
Rubraca is currently approved for the treatment of certain patients with ovarian and prostate cancer. Several label expansion studies are ongoing on Rubraca.
The phase III ATHENA study is evaluating Rubraca as a monotherapy and in combination with
Bristol Myers
’
BMY
Opdivo for advanced ovarian cancer as a first-line maintenance treatment.
In March 2022, Clovis reported positive top-line data from the monotherapy arm of the ATHENA study (ATHENA-MONO), which achieved its primary endpoint of a statistically significant progression-free survival (PFS) versus placebo. Based on this data, CLVS intends to seek label expansion for Rubraca as a first-line ovarian cancer maintenance treatment in both the United States and Europe.
Top-line data from the combination arm of the ATHENA study (ATHENA-COMBO) is anticipated in first-quarter 2023. The ATHENA study is part of a broader clinical collaboration with Bristol Myers, finalized in 2017. Opdivo is one of the key drivers of Bristol Myers’s top line. During first-quarter 2022, which ended on Mar 31, BMY recorded $1.9 billion from Opdivo sales.
A confirmatory phase III TRITON3 study is evaluating Rubraca in metastatic castration-resistant prostate cancer (mCRPC) patients with tumors associated with BRCA mutations and ATM mutations. Data from this study is expected in third-quarter 2022. This will serve as a confirmatory study for the continued approval of Rubraca to treat mCRPC. It will also serve as a potential second-line label expansion for Rubraca to address mCRPC.
FAP-2286
Clovis is currently evaluating FAP-2286, its lead peptide-targeted radionuclide therapy (PTRT) and imaging agent candidate, in the phase I/II LuMIERE study across multiple tumor types.
While the phase I portion of the study will determine the dose and tolerability of the candidate, the phase II portion will consist of the expansion cohorts to be evaluated for multiple tumor types. CLVS expects to present the initial data from the phase I portion of the study next month at a medical meeting. Management also intends to start the phase II portion of the study in fourth-quarter 2022.
Zacks Rank & Other Stocks to Consider
Clovis currently carries a Zacks Rank #2 (Buy). Other top-ranked stocks in the overall healthcare sector include
Deciphera Pharmaceuticals
DCPH
and
Vertex Pharmaceuticals
VRTX
, each carrying a Zacks Rank #2 at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Deciphera Pharmaceuticals’ loss per share estimates for 2022 have narrowed from $2.94 to $2.73 in the past 30 days. The same for 2023 has narrowed from $2.38 to $1.84 in the same time period. DCPH has risen 8% in the year-to-date period.
Earnings of Deciphera Pharmaceuticals missed estimates in three of the last four quarters and beat the mark once, the average negative surprise being 2.7%.
Vertex Pharmaceuticals’ earnings per share estimates for 2023 have increased from $15.31 to $15.35 in the past 30 days. VRTX has rallied 22.6% in the year so far.
Earnings of Vertex Pharmaceuticals beat estimates in each of the last four quarters, the average being 10%.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









