Shares of
Novavax
NVAX
were up nearly 20% in pre-market trading on Jun 8. This upside was led by management’s announcement that NVAX has received a positive recommendation from the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) for its protein-based COVID-19 vaccine NVX-CoV2373.
The VRBPAC voted 21-0 (with one abstention), recommending the FDA to grant emergency use authorization (EUA) to NVX-CoV2373 for use in adults aged 18 years and older. If granted an EUA by the regulatory body, Novavax’s COVID-19 vaccine will be the first protein-based shot available in the United States.
A tentative authorization to NVX-CoV2373 will also help NVAX achieve its desired financial guidance for 2022. Management expects to record total revenues for the full year between $4 billion and $5 billion.
Shares of Novavax have plunged 66.8% so far this year compared with the
industry
’s 23.2% decline.
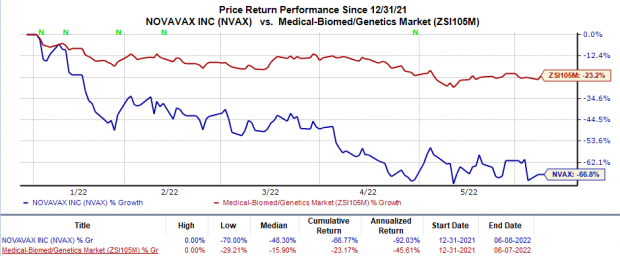
Image Source: Zacks Investment Research
The FDA filing seeking an EUA for the COVID-19 vaccine was
submitted
by NVAX in January this year. The filing is based on data from the two pivotal phase III studies on the vaccine, one (PREVENT-19) conducted in the United States and Mexico, while another in the United Kingdom. The PREVENT-19 study achieved an overall vaccine efficacy of 90.4%, while data from the study conducted in the United Kingdom demonstrated that the vaccine achieved an overall efficacy of 89.7%.
Novavax also submitted an amendment to its earlier regulatory filing with the FDA. This amendment provides an updated piece of manufacturing information on NVX-CoV2373 to the regulatory body for review.
Notably, the Novavax vaccine is already authorized for use in more than 40 countries, including Australia, Canada, the European countries and India. NVAX is currently marketing two versions of NVX-CoV2373, one marketed in partnership with the Serum Institute of India under the trade name Covovax while the other is produced by itself and marketed under the trade name Nuvaxovid.
Once approved, NVX-CoV2373 will face stiff competition from the COVID-19 vaccines developed by
Moderna
MRNA
and
Pfizer
PFE
/
BioNTech
BNTX
. Novavax is already trailing a lot behind these vaccines. These vaccines dominate the U.S. market and are the only ones to have received full approval for use in adults in the country.
The two vaccines developed by Moderna and Pfizer/BioNTech are based on the mRNA technology with high efficacy rates. In fact, the booster doses of these vaccines are also authorized for use in adults.
The primary regimen of Pfizer/BioNTech’s COVID vaccine is currently the only one authorized for use in individuals, aged five years and above in the United States. The third dose of the Pfizer/BioNTech vaccine is also the only shot allowed for use in the individuals aged 12 years and above.
Although Moderna’s vaccine is yet to be authorized for use in individuals under the age of 18 years (either as a primary regimen or a booster dose) in the United States, its management already submitted regulatory applications to this end, which are currently under the FDA review.
Additionally, the FDA authorized the use of a second booster dose of Moderna and Pfizer/BioNTech vaccines in older individuals (aged 50 years and beyond) as well as in certain immunocompromised individuals, aged 18 years and above.
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Zacks’ Top Picks to Cash in on Electric Vehicles
Big money has already been made in the Electric Vehicle (EV) industry. But, the EV revolution has not hit full throttle yet. There is a lot of money to be made as the next push for future technologies ramps up. Zacks’ Special Report reveals 5 picks investors
See 5 EV Stocks With Extreme Upside Potential >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









