Denmark-based biotech
Genmab A/S
GMAB
received Orphan Drug designation for its antibody product candidate, epcoritamab, for treating follicular lymphoma (“FL”) from the FDA.
The orphan drug designation is granted by the FDA to a drug or biologic intended to treat a rare disease or condition, which generally includes a disease or condition that affects fewer than 200,000 individuals in the United States. The orphan drug designation also includes incentives including tax credits for clinical testing, prescription drug user fee exemptions and a seven-year marketing exclusivity in the event of regulatory approval.
Please note that Genmab is co-developing the candidate with
AbbVie
ABBV
. Genmab and AbbVie inked an oncology collaboration in 2020 to jointly develop and market three of Genmab’s early-stage investigational bispecific antibody product candidates including epcoritamab. AbbVie and Genmab share commercial responsibilities for epcoritamab in the United States and Japan while AbbVie is responsible for other regions.
Genmab and AbbVie are evaluating epcoritamab in three early- to mid-stage clinical studies for treating FL. A phase I/II study — EPCORE — is evaluating a subcutaneous formulation of Genmab and AbbVie’s candidate in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma (B-NHL), which includes diffuse large B-cell Lymphoma (DLCBL), FL, and mantle cell lymphoma (MCL). Another phase I/II study is evaluating the safety and efficacy of epcoritamab in Japanese patients with relapsed/refractory B-NHL. A phase Ib/II study is evaluating epcoritamab in combination with standard of care across different lines of therapy in patients with DLBCL or FL.
Shares of Genmab have declined 19.6% so far this year compared with the
industry
’s decrease of 19.4%.
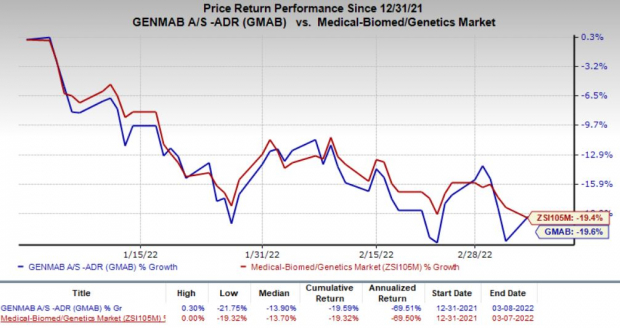
Image Source: Zacks Investment Research
We note that epcoritamab has been developed using Genmab’s proprietary DuoBody technology platform. Apart from AbbVie, the company’s technology platform has also attracted
J&J
JNJ
to sign an agreement. In 2012, J&J and Genmab entered into a partnership for developing bispecific antibodies using the latter’s proprietary DuoBody technology platform. In May 2021, J&J received FDA approval for Rybrevant, developed using Genmab’s DuoBody technology platform, for treating adult patients with non-small cell lung cancer with EGFR mutations. J&J’s Darzalex and Darzalex Faspro have also been developed in partnership with Genmab.
Last year, Genmab’s partner
Seagen
SGEN
announced that the FDA had granted
accelerated approval
to their investigational antibody-drug conjugate, Tivdak (tisotumab vedotin-tftv). Seagen received approval for Tivdak for recurrent/metastatic cervical cancer in adult patients whose disease progressed on or after chemotherapy. Seagen and Genmab continue to evaluate Tivdak as a potential treatment for cervical cancer and other solid tumors in different clinical studies.
Zacks Rank
Genmab currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









