Gilead Sciences, Inc
.
GILD
announced that the European Medicines Agency (EMA) has validated a Type II variation Marketing Authorization Application (MAA) for Trodelvy (sacituzumab govitecan-hziy).
The MAA is seeking approval of the drug for the treatment of adult patients with unresectable or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting.
The application is based on data from the registrational phase III TROPiCS-02 study, which achieved its primary endpoint of progression-free survival (PFS) and key secondary endpoint of overall survival (OS) versus comparator chemotherapies (treatment of physician’s choice (TPC) of chemotherapy).
Last October, the FDA accepted for priority review the supplemental biologics license application (sBLA) for Trodelvy for the treatment of adult patients with unresectable locally advanced or metastatic HR+/HER2-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting. The target action date is currently set for February 2023.
Please note that Trodelvy is approved in more than 40 countries with multiple additional regulatory reviews underway worldwide for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Trodelvy is also approved in the United States under the accelerated approval pathway for the treatment of adult patients with locally advanced or metastatic urothelial cancer (UC) who have previously received a platinum-containing chemotherapy and either a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor.
The uptake of the breast cancer drug Trodelvy has been strong and has boosted the top line. Approval in additional geographies and indications will further fuel the growth potential of the drug.
The oncology space is lucrative and Gilead can capitalize on it, thereby creating a revenue growth driver in addition to the HIV franchise.
Shares have gained 18.3% in the year so far against the
industry
’s decline of 20.9%.
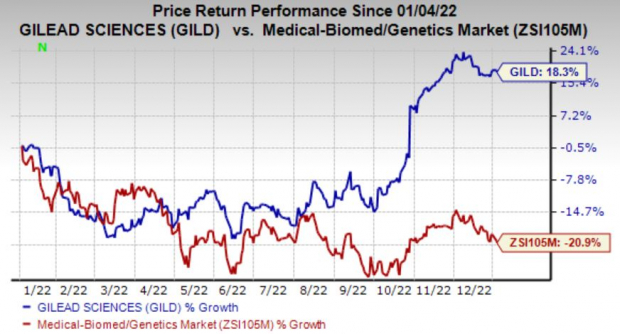
Image Source: Zacks Investment Research
Gilead also announced a collaboration and license agreement with EVOQ Therapeutics to advance EVOQ’s proprietary technology for the treatment of rheumatoid arthritis (RA) and lupus.
Per the terms, Gilead and EVOQ will collaborate on preclinical development. Gilead has the option to exclusively license rights to EVOQ’s NanoDisc technology to pursue product candidates for RA and lupus indications and will be responsible for clinical development and commercialization.
The company is focusing on strategic collaborations to bolster its pipeline. Last week, Gilead acquired the rights to GS-1811 (formerly JTX-1811) from
Jounce Therapeutics
JNCE
. Both companies amended their existing license agreement for GS-1811, which will enable Gilead to buy out any remaining contingent payments potentially due under the license agreement executed in August 2020. As part of the transaction, certain operational obligations of the parties related to GS-1811 set forth in the license agreement have also been terminated.
Gilead will be solely responsible for all further research, development and commercialization of GS-1811 globally. In exchange, Jounce will receive proceeds of $67 million for this transaction but will no longer be entitled to receive the remaining contingent payments of up to $645 million in milestones and high single-digit to mid-teens royalties based upon worldwide sales under the original license agreement.
The company earlier announced a global strategic collaboration with a clinical-stage biotechnology company,
Arcellx, Inc.
ACLX
, to co-develop and co-commercialize the latter’s lead late-stage product candidate, CART-ddBCMA. Per the terms, Arcellx will receive an upfront cash payment of $225 million and a $100 million equity investment, as well as other potential contingent payments. Gilead’s wholly owned subsidiaries, Kite and Arcellx, will share development, clinical trial and commercialization costs for CART-ddBCMA and will jointly commercialize the product. The profits of the deal will be split equally. The transaction is expected to close in the first quarter of 2023.
Gilead currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









