Eli Lilly
LLY
and partner
Incyte
INCY
announced two important updates related to its oral, once-daily JAK1 inhibitor Olumiant (baricitinib) in systemic lupus erythematosus (SLE or lupus) and atopic dermatitis (ADor eczema) indications.
Lilly and Incyte have decided to discontinue the pivotal phase III development program evaluating Olumiant in lupus. The decision is based on data from two pivotal phase III studies — SLE-BRAVE-I and SLE-BRAVE-II — evaluating the drug against placebo across 1500 adult participants with active lupus. While the SLE-BRAVE-I study met its primary endpoint, the SLE-BRAVE-II study failed to achieve the same. In fact, neither of the studies achieved secondary endpoint.
The primary endpoint of both SLE-BRAVE-I and SLE-BRAVE-II studies was to measure the proportion of active lupus patients who achieved an SRI-4 response at week 52. SRI-4 is a composite clinical endpoint to measure the response of treatment using both Olumiant and placebo by comparing the decline in overall disease activity.
Eli Lilly plans to analyze the data from the lupus studies and publish the same at a later date. Currently, Lilly is working with investigators to settle the SLE-BRAVE-X study, a phase III long-term extension study evaluating Olumiant over a period of three years in patients who completed the SLE-BRAVE-I and SLE-BRAVE-II studies.
Shares of Eli Lilly have risen 20.7% in the trailing 12 months in comparison with the
industry
’s 17.5% rise.
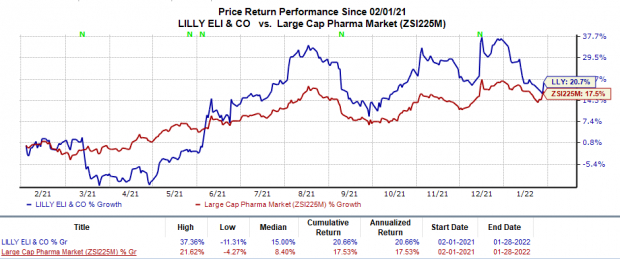
Image Source: Zacks Investment Research
Incyte’s stock has declined 18.4% in the past year in comparison with the
industry
’s 39.2% decline.
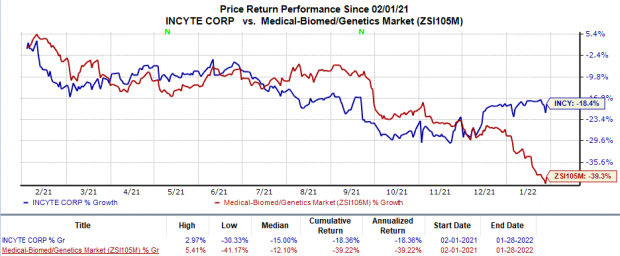
Image Source: Zacks Investment Research
Both Eli Lilly and Incyte also announced an update to their supplemental new drug application (sNDA) with the FDA seeking label expansion for Olumiant in adults with moderate-to-severe AD.
Per the press release, both Eli Lilly and Incyte expect the FDA to issue a complete response letter (CRL) to the sNDA, as Eli Lilly does not have alignment with the FDA on the indicated population.
Please note that Olumiant is already approved in more than 50 countries, including the European Union and Japan, to treat AD in adults who are candidates for systemic therapy.
Currently, Olumiant is approved in the United States for the treatment of adults with moderate-to-severe rheumatoid arthritis (RA). Barictinib is also authorized by the FDA for emergency use in certain hospitalized COVID patients. The FDA is currently reviewing the sNDA seeking full approval in the given indication and a decision is expected in the second quarter.
Please note that
AbbVie
ABBV
and
Pfizer
PFE
gained FDA approval for their JAK inhibitor drugs in the United States to treat AD.
Earlier this month, AbbVie
announced
that the FDA granted label expansion to its JAK inhibitor, Rinvoq, for the treatment of moderate-to-severe AD in adults and children aged 12 years and above who did not respond to treatment with other drugs or when the use of other treatments is not recommended.
This month, Pfizer also
announced
that the FDA approved its JAK1 inhibitor, Cibinqo (abrocitinib), for the treatment of refractory moderate-to-severe AD in adult patients who did not respond to treatment with other drugs or when the use of other treatments is not recommended.
We note that the FDA remains skeptical toward the JAK inhibitor drugs. Last September, the FDA issued a Drug Safety Communication to include warnings about an increased risk of serious heart-related events, cancer, blood clots and even death, to be added to the label of three JAK inhibitor drugs — including Olumiant, AbbVie’s Rinvoq and Pfizer’s another JAK inhibitor drug, Xeljanz — that are already approved for RA indication.
This FDA decision was based on its review of the final data from the post-marketing safety study of Pfizer’s Xeljanz in RA indication. The FDA limited the use of Xeljanz to certain patients who have not responded to or cannot tolerate one or more TNF blockers. Since AbbVie’s Rinvoq and Olumiant also have a similar mechanism for treatment, the directive was extended to them as well.
Zacks Rank
Eli Lilly currently carries a Zacks Rank #2 (Buy) while Incyte holds a Zacks Rank #4 (Sell) at present.
You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
As one investor put it, “curing and preventing hundreds of diseases…what should that market be worth?” This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report







