Merck & Co.
MRK
announced that the FDA has granted approval to its blockbuster PD-L1 inhibitor, Keytruda plus partner Eisai’s Lenvima for the first-line treatment of patients with advanced renal cell carcinoma (RCC), a form of kidney cancer.
The approval for this promising combination option in the first-line setting is based on data from the pivotal phase III CLEAR (Study 307)/KEYNOTE-581 study. In the study, Keytruda+Lenvima demonstrated statistically significant and clinically meaningful improvements in progression-free survival (PFS) and overall survival (OS) versus
Pfizer
’s
PFE
Sutent (sunitinib). For PFS, the data showed that Keytruda plus Lenvima significantly reduced the risk of disease progression or death by 61% versus Sutent (sunitinib). The median PFS for the Keytruda plus Lenvima arm was nearly two years versus little more than nine months for Sutent. For OS, Keytruda plus Lenvima reduced the risk of death by 34% versus Sutent.
At present, Keytruda is approved in combination with Pfizer’s Inlyta (axitinib) for first-line treatment of advanced RCC. Keytruda is being evaluated across 20 studies for RCC. Earlier this week, the FDA accepted and granted priority review to Merck’s supplemental biologics license application (sBLA) seeking approval of Keytruda for the adjuvant treatment of RCC patients at intermediate-high or high risk of recurrence following nephrectomy (surgical removal of a kidney) or following nephrectomy and resection of metastatic lesions. The FDA’s decision is expected on Dec 10, 2021. This sBLA was based on data from the pivotal phase III KEYNOTE-564study.
Keytruda plus Lenvima is now approved for two types of cancers, RCC and advanced endometrial carcinoma that is not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR). Merck has a deal with Japan’s Eisai to co-develop and commercialize Lenvima, both as a monotherapy as well as in combination with Keytruda, for several types of cancers.
Merck’s stock has declined 8.1% this year so far against an increase of 15.6% for the
industry
.
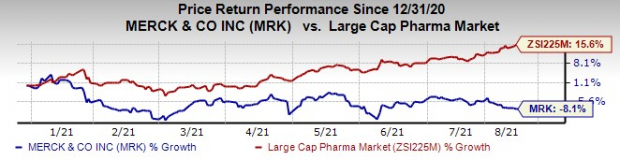
Image Source: Zacks Investment Research
Keytruda is a key top-line driver for Merck as it is approved for the treatment of many cancers globally. The drug generated sales of $4.18 billion in the second quarter of 2021, up 20% (excluding Fx impact) year over year.
Keytruda is continuously growing and expanding into new indications and markets globally. In fact, the Keytruda development program is also progressing well and the drug is being studied for more than 30 types of cancer in more than 1550 studies, including more than 1100 combination studies. Merck has collaborated with several companies including
Amgen
AMGN
,
Glaxo
GSK
and Pfizer separately for the evaluation of Keytruda in combination with other regimens.
Merck currently carries a Zacks Rank #4 (Sell).
You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Bitcoin, Like the Internet Itself, Could Change Everything
Blockchain and cryptocurrency has sparked one of the most exciting discussion topics of a generation. Some call it the “Internet of Money” and predict it could change the way money works forever. If true, it could do to banks what Netflix did to Blockbuster and Amazon did to Sears. Experts agree we’re still in the early stages of this technology, and as it grows, it will create several investing opportunities.
Zacks’ has just revealed 3 companies that can help investors capitalize on the explosive profit potential of Bitcoin and the other cryptocurrencies with significantly less volatility than buying them directly.
See 3 crypto-related stocks now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








