Mirati Therapeutics
MRTX
announced that it has filed a marketing authorization application (MAA) in the European Union (EU) for its KRAS-G12C inhibitor adagrasib for non-small cell lung cancer (NSCLC).
The MAA filed with the European Medicines Agency (EMA) seeks approval for adagrasib as a potential treatment for patients with KRAS-G12C-mutated NSCLC. The patients must have received at least one prior systemic therapy.
MRTX already submitted a new drug application (NDA) with the FDA seeking approval under the accelerated pathway for adagrasib for the above-mentioned indication. In February, Mirati announced that the FDA
accepted
the NDA and set a PDUFA action date of Dec 14, 2022, for the same.
Shares of Mirati have plunged 59.4% in the year-to-date period compared with the
industry
’s 24.1% decline.
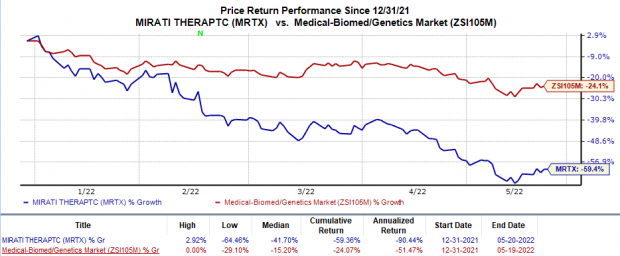
Image Source: Zacks Investment Research
The filings in both the United States and the EU are based on positive top-line data from the phase II potentially registration-enabling monotherapy-cohort of the KRYSTAL-1 study, evaluating adagrasib for the above-mentioned indication. Data from the study was announced last Sepetmber. In the study, treatment with adagrasib led to an objective response rate of 43% and a disease control rate of 80% as of Jun 15, 2021.
Currently, there are very limited options available to patients suffering KRAS-G12C mutated NSCLC. A potential approval of adagrasib will provide Mirati with its first marketed drug and the patients with a new therapeutic option.
Mirati’s adagrasib, if approved, will face stiff competition from Lumakras, a KRAS-G12C inhibitor, marketed by
Amgen
AMGN
. AMGN received accelerated approval for Lumakras as a second-line treatment for locally advanced or metastatic NSCLC from the FDA last May. Earlier in January this year, Amgen also gained approval for the drug in Europe for a similar indication. The drug has shown robust launch uptake since its approval, with Amgen recording revenues worth $62 million from its sales in first-quarter 2022.
The KRYSTAL-1 study is also evaluating adagrasib in multiple cohorts in combination with other therapies. These include a combo therapy of adagrasib with
Merck
’s
MRK
Keytruda for first-line NSCLC, a combination of adagrasib plus Boehringer Ingelheim’s Gilotrif (afatinib) for advanced NSCLC and adagrasib combined with
Bristol-Myers
’
BMY
Erbitux for second-line colorectal cancer (CRC).
Preliminary data from the adagrasib plus Merck’s Keytruda cohort demonstrated that the combination achieved a 100% disease control rate, with all seven patients exhibiting tumor regression ranging from 37% to 92% as of Oct 21, 2021.
Mirati is also pursuing a broad combination development program for adagrasib beyond the combinations with Merck’s Keytruda and Bristol-Myers’ Erbitux. These include combinations with SHP2, SOS1 or CDK 4/6 inhibitors.
Both Opdivo and Keutruda are key drivers for Bristol Myers and Merck’s top lines, respectively. During the first quarter of 2022, Bristol Myers recorded $1.9 billion from Opdivo sales while Merck recorded $4.8 billion from Keytruda sales.
Zacks Rank
Mirati currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









