Moderna
MRNA
announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (“CHMP”) recommended label expansion for mRNA-1273.214, its mRNA-based bivalent BA.1 Omicron-targeting COVID-19 vaccine, for use in children aged six through 11 years.
The CHMP recommends that mRNA-1273.214 be administered to children at least three months after the last prior dose of a COVID-19 vaccine.
Along with mRNA-1273.214, Moderna also markets mRNA-1273.222, another bivalent targeting the Omicron BA.4/5 subvariants in Europe. Both bivalent vaccines are currently authorized for adults and adolescents aged 12 years and older.
The latest CHMP recommendation is supported by data based on clinical studies which evaluated a third/booster dose of Spikevax in participants aged between six through 11 years who already had completed a primary series of Moderna’s COVID vaccine. The recommendation is also based on data from a phase II/III study that evaluated mRNA-1273.214 in adults aged 18 years and older. Last month, management
announced
that this phase II/III study achieved its primary endpoint of generating a superior antibody response in study participants compared with the booster dose of Spikevax. The bivalent vaccine also met non-inferiority immunogenicity criteria to the original strain.
Moderna is also advancing a phase II/III study evaluating mRNA-1273.214 as booster and primary series in children six months through five years. Initial data from the study are expected in early 2023.
Shares of Moderna have declined 23.9% so far this year compared with the
industry
’s 19.7% fall.
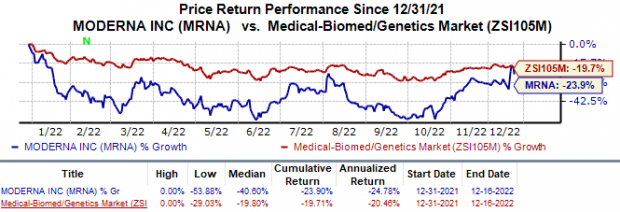
Image Source: Zacks Investment Research
The bivalent vaccine contains an mRNA encoding the spike protein in the original/monovalent vaccine and an mRNA encoding the spike protein common in the Omicron variants. While mRNA-1273.222 contains the spike protein in the BA.4/5 Omicron subvariants, mRNA-1273.214 contains the spike protein in the BA.1 Omicron subvariant.
Moderna faces stiff competition from
Pfizer
PFE
/
BioNTech
BNTX
, who also market their bivalent COVID-19 vaccines in the European Union. Pfizer/BioNTech currently markets two COVID-19 bivalent vaccines — one targeting the BA.1 subvariant and another targeting the BA.4/5 subvariant. While Pfizer/BioNTech’s BA.1 targeting bivalent vaccine is authorized for use in individuals aged 12 years and older, their BA.4/5 vaccine is authorized for use in people aged five years and older.
In the United States, Moderna and Pfizer/BioNTech’s respective bivalent Omicron BA.4/5-targeting COVID vaccines are authorized for use in individuals aged six months and older.
Zacks Rank & Stocks to Consider
Moderna currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is
Kamada
KMDA
, which sports a Zacks Rank #1 (Strong Buy) at present. You can see
the complete list of today’s Zacks #1 Rank stocks here
.
In the past 30 days, estimates for Kamada’s 2022 loss per share have narrowed from 14 cents to 7 cents. During the same period, the earnings estimates per share for 2023 have risen from 26 cents to 42 cents. Shares of Kamada have declined 40.9% in the year-to-date period.
Earnings of Kamada beat estimates in two of the last four quarters and missed the mark twice, witnessing a negative earnings surprise of 62.50%, on average. In the last reported quarter, Kamada’s earnings beat estimates by 433.33%.
Special Report: The Top 5 IPOs for Your Portfolio
Today, you have a chance to get in on the ground floor of one of the best investment opportunities of the year. As the world continues to benefit from an ever-evolving internet, a handful of innovative tech companies are on the brink of reaping immense rewards – and you can put yourself in a position to cash in. One is set to disrupt the online communication industry. Brilliantly designed for creating online communities, this stock is poised to explode when made public. With the strength of our economy and record amounts of cash flooding into IPOs, you don’t want to miss this opportunity.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









