MorphoSys AG
MOR
along with
Incyte
INCY
and
Pfizer
PFE
announced a clinical trial collaboration and supply agreement to develop a combination therapy of MorphoSys and Incyte’s cancer drug, Monjuvi, and Pfizer’s checkpoint inhibitor candidate, TTI-622. The companies are planning to develop the combination therapy plus
Bristol-Myers
’
BMY
Revlimid (lenalidomide) in patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) who are not eligible for autologous stem cell transplantation (ASCT).
Please note that MorphoSys’ Monjuvi is approved in combination with Bristol-Myers’ Revlimid for treating adult patients with relapsed or refractory DLBCL not otherwise specified, including DLBCL arising from low-grade lymphoma, and those who are not eligible for ASCT. Bristol-Myers’ Revlimid is approved for several blood cancer indications, including mantle cell lymphoma and follicular lymphoma.
MorphoSys co-commercializes Monjuvi with Incyte in the United States. INCY has exclusive commercialization rights to the drug outside the United States.
Per the terms of the agreement, Pfizer will initiate a phase Ib/II study to evaluate the combination of TTI-622 with Monjuvi and lenalidomide in the r/r DLBCL patients not eligible for ASCT. The global study will be sponsored and funded by Pfizer. MorphoSys and Incyte will supply Monjuvi for the study.
Shares of MorphoSys gained more than 14% in after-hours trading on Jun 13 as the deal with Pfizer may prove to be beneficial for Monjuvi. However, shares of the company have declined 50.9% so far this year compared with the
industry
’s decrease of 27.5%.
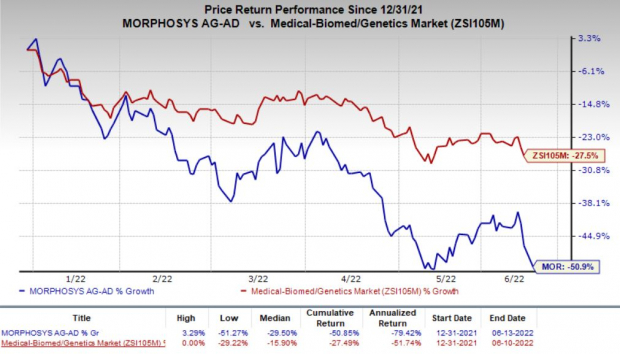
Image Source: Zacks Investment Research
MorphoSys believes that the addition of novel immunotherapies like TTI-622 to the Monjuvi plus Revlimid therapy may lead to a new meaningful combination treatment option for r/r DLBCL patients. The company’s partner Incyte believes that there is a significant unmet medical need in this patient population.
Pfizer is currently evaluating TTI-622, an anti-CD47 blocking agent, in a phase Ib/II study as a monotherapy targeting several indications, with a focus on hematological malignancies. The candidate helps in blocking CD47, an innate immune checkpoint that is overexpressed in solid and hematological malignancies, including in DLBCL.
Early study data demonstrated that TTI-662 has the potential to be a class-leading monotherapy in late-line advanced lymphoid malignancies. Pre-clinical data provides evidence that the addition of TTI-622 to a diverse set of therapeutic agents may lead to prospective therapies. The collaboration with MorphoSys and Incyte will help Pfizer to gather additional evidence on the potential of TTI-622 to improve outcomes for patients with DLBCL.
Moreover, data from preclinical studies conducted by MorphoSys have shown a strong synergy of Monjuvi and anti-CD47 antibodies.
Zacks Rank
MorphoSys currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
How to Profit from the Hot Electric Vehicle Industry
Global electric car sales in 2021 more than doubled their 2020 numbers. And today, the electric vehicle (EV) technology and very nature of the business is changing quickly. The next push for future technologies is happening now and investors who get in early could see exceptional profits.
See Zacks’ Top Stocks to Profit from the EV Revolution >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









