Novartis
NVS
has announced that the FDA granted accelerated approval to the combination of Tafinlar (dabrafenib) + Mekinist (trametinib) for the treatment of adult and pediatric patients six years of age and older with unresectable or metastatic solid tumors with BRAF V600E mutation who have progressed following prior treatment and have no satisfactory alternative treatment options.
The FDA approval was supported by results from phase II ROAR and NCI-MATCH studies demonstrating overall response rates up to 80% in patients with BRAF V600E solid tumors.
Per the company, Tafinlar + Mekinist is the first and only therapy to be approved with a tumor-agnostic indication for adult and pediatric patients with solid tumors that have a BRAF V600E mutation.
Tafinlar + Mekinist is already approved for use in BRAF V600 mutation-positive unresectable or metastatic melanoma, as an adjuvant treatment for BRAF V600 mutation-positive melanoma after surgery, in BRAF V600 mutation-positive metastatic non-small cell lung cancer (NSCLC) and in BRAF V600 mutation-positive anaplastic thyroid cancer.
Sales from Tafinlar + Mekinist came in at $403 million in the first quarter.
In addition, the European Commission approved the lung cancer drug Tabrecta (capmatinib). It was approved as a monotherapy for the treatment of adults with advanced NSCLC harboring alterations leading to mesenchymal-epithelial-transition factor gene (MET) exon 14 (METex14) skipping who require systemic therapy following prior treatment with immunotherapy and/or platinum-based chemotherapy.
Approval was in the cards as the Committee for Medicinal Products for Human Use of the European Medicines Agency issued a positive opinion on the same in April.
Novartis had in-licensed Tabrecta from
Incyte Corporation
INCY
in 2009. Tabrecta was discovered by Incyte. Incyte granted Novartis worldwide exclusive development and commercialization rights to capmatinib. INCY is eligible to receive royalties on net sales of Tabrecta.
Approval of new drugs and label expansion of existing drugs are likely to boost Novartis’ top line in the forthcoming quarters.
Shares of Novartis have lost 6.7% so far this year compared with the
industry
’s growth of 1.3%.
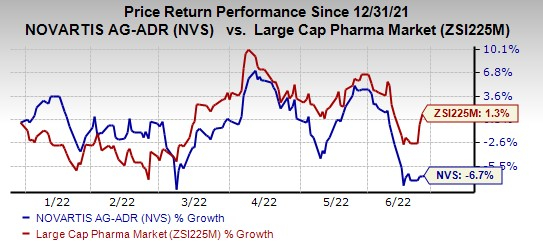
Image Source: Zacks Investment Research
Novartis’ performance in the first quarter was good as the lagging Sandoz business returned to growth and cardiovascular drug Entresto maintained its stellar performance.
Drugs like Cosentyx, Entresto, Kesimpta, Zolgensma, Kisqali and Leqvio should continue to fuel growth and offset the impact of generic competition.
Novartis currently carries a Zacks Rank of #3 (Hold). Some better-ranked stocks are
Alkermes
ALKS
and
Geron Corporation
GERN
, both carrying a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
ALKS’ loss estimates for 2022 have narrowed to 3 cents from a loss of 14 cents in the past 60 days. Alkermes surpassed earnings in all the trailing four quarters, the average being 350.48%.
GERN’s loss estimates for 2022 have narrowed 7 cents in the past 60 days. Geron surpassed on earnings in three of the trailing four quarters and missed the mark in one, the average surprise being 1.07%.
Zacks’ Top Picks to Cash in on Electric Vehicles
Big money has already been made in the Electric Vehicle (EV) industry. But, the EV revolution has not hit full throttle yet. There is a lot of money to be made as the next push for future technologies ramps up. Zacks’ Special Report reveals 5 picks investors
See 5 EV Stocks With Extreme Upside Potential >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








