Novavax
NVAX
announced that it has secured an emergency use authorization (EUA) in India for its protein-based COVID-19 vaccine NVX-CoV2373 in adolescents, aged from 12 years to 17 years.
With this EUA, NVX-CoV2373 receives its first authorization for use in the adolescent population.
The authorization in India was secured by Novavax’s partner Serum Institute of India (Serum), which manufactures and markets a version of NVX-CoV2373 under the trade name Covovax. Covovax was
granted
an EUA last December in India for use in adults aged 18 years and above. Following the EUA approval of adolescents, the vaccine is now authorized for use in individuals aged 12 years and above in the country.
Shares of Novavax have plunged 41.4% in the year-to-date period compared with the
industry
’s 13.7% decline.
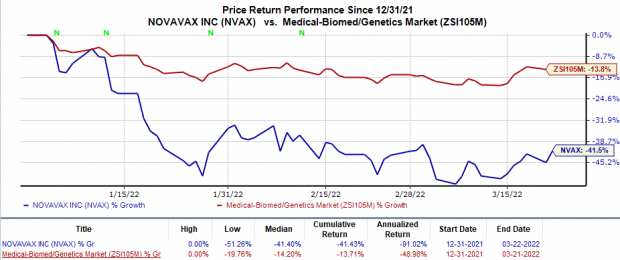
Image Source: Zacks Investment Research
The EUA granted by the Indian health regulator was based on results from a phase II/III study conducted in India, which evaluated Covovax in 460 adolescents. Data from the study demonstrated the safety and immunogenicity of the COVID vaccine in the age group of 12-17 years. Both NVAX and Serum are also conducting studies in India in younger kids, aged 2-11 years.
The above results were also consistent with the data
reported
by Novavax from the ongoing phase III PREVENT-19 pediatric expansion study last month. The pediatric expansion study achieved its primary effectiveness endpoint of NVX-CoV2373 generating neutralizing antibodies in adolescents, similar to the antibody responses in young adult patients (aged between 18 years and 26 years), who were administered the vaccine in the phase III PREVENT-19 study.
Apart from India, NVX-CoV2373 is also authorized for emergency use in adults in other highly populated markets like Australia, Canada, Europe and Indonesia. However, the vaccine is yet to be authorized in the United States. Novavax’s EUA
filing
with the FDA, which seeks authorization for use of NVX-CoV2373 in adults, is currently under review. NVAX also submitted regulatory filings seeking authorizations for use of NVX-CoV2373 in Japan and South Africa.
Although Novavax’s COVID vaccine shows promise and is currently garnering approvals across multiple nations, it is lagging
Moderna
MRNA
and
Pfizer
PFE
/
BioNTech
BNTX
, which developed their own COVID vaccines that not only dominate the market in the United States but also other markets across the globe.
We note that the vaccines developed by Moderna and Pfizer/BioNTech are based on the mRNA technology and require a two-dose primary regimen. In fact, those COVID vaccines are currently the only ones that received full approval for use in adults in the United States.
The primary regimen of Pfizer/BioNTech’s COVID vaccine is already authorized in the United States as well as many other countries for emergency use in adolescents. Although Moderna’s COVID vaccine is yet to be granted an EUA in the United States for use in adolescents, the same is already authorized for use in adolescents in many countries, including Europe and Canada.
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Investor Alert: Legal Marijuana Looking for big gains?
Now is the time to get in on a young industry primed to skyrocket from $13.5 billion in 2021 to an expected $70.6 billion by 2028.
After a clean sweep of 6 election referendums in 5 states, pot is now legal in 36 states plus D.C. Federal legalization is expected soon and that could kick start an even greater bonanza for investors. Zacks Investment Research has recently closed pot stocks that have shot up as high as +147.0%.
You’re invited to immediately check out Zacks’
Marijuana Moneymakers: An Investor’s Guide
. It features a timely Watch List of pot stocks and ETFs with exceptional growth potential.
Today, Download Marijuana Moneymakers FREE >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









