Pfizer
PFE
and its Germany-based partner
BioNTech
’s
BNTX
mRNA-based COVID-19 vaccine elicited a strong immune response, high efficacy and a favorable safety profile when given as a three-dose vaccine to children six months to under five years of age
Top-line data from the phase II/III study showed that the three-dose vaccine was as effective in the 6- to 24-month-old population and the 2- to under 5-year-old population as the second dose in the 16- to 25-year-old population. The vaccine’s efficacy was 80.3% in the above-mentioned age group. This vaccine efficacy, a secondary endpoint in the study, was observed in the descriptive analysis of three doses while the Omicron variant remained dominant.
Pfizer’s formulation of a booster dose for these youngest children is 3-µg, which is one-tenth of the dose strength for adults.
Pfizer and BioNTech’s COVID vaccine is not yet authorized for use in children six months to under five years of age even as a two-dose primary series. The companies initiated a rolling submission for Emergency Use Authorization (EUA) for a two-dose primary series of the vaccine in this age group in February. Based on the latest data, Pfizer and BioNTech will submit the latest safety, immunogenicity, and vaccine efficacy data for the third dose as part of the same rolling submission in this age group later this week.
Last year in December, a pre-specified immunogenicity analysis of data from the phase II/III study in children aged six months to five years showed that the COVID-19 vaccine, as a two-dose regimen, failed to demonstrate non-inferior immunogenicity levels in two to five years age group compared to 16 to 25 years aged individuals. Back then, Pfizer and BioNTech decided to test a third 3 µg dose of their COVID-19 vaccine in children aged six months to under five years for better protection.
Rival
Moderna
’s
MRNA
mRNA-based vaccine is also not yet authorized for use in kids under 6 years of age. Moderna has already filed data with the FDA seeking emergency approval for two 25-µg doses of its vaccine in children six months to under six years of age. Its two-dose vaccine was 37%-51% effective in this age group in an analysis when the Omicron variant was circulating.
The FDA is due to meet next month to review Pfizer and BioNTech and Moderna’s authorization requests for kids aged six months to 5 years and six months to 6 years.
Pfizer’s stock is down 10.5% this year so far against an increase of 4.5% for the
industry
.
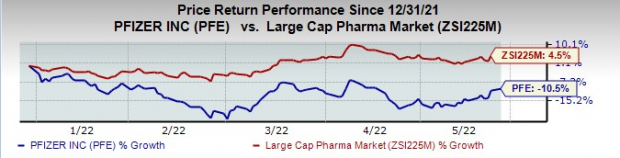
Image Source: Zacks Investment Research
Germany-based BioNTech’s shares are down 36.6% this year so far compared with the
industry
’s decrease of 23.7%.
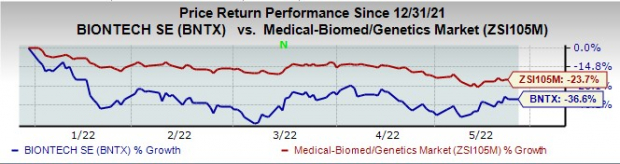
Image Source: Zacks Investment Research
Pfizer and BioNTech and Moderna’s first booster doses of their vaccines were granted EUA for all adults 18 years of age and older in November 2021. Later, Pfizer’s EUA was extended to allow its use in individuals 16 and 17 years of age and thereafter in adolescents 12 to 15 years of age and finally in kids 5 through 11 years of age last week.
In March this year, the FDA authorized a second booster dose of Pfizer and BioNTech and Moderna’s vaccines for older adults and some immunocompromised people. Pfizer and BioNTech and Moderna’s booster shots are authorized for use in adults in Europe.
A booster shot of
J&J
’s
JNJ
adenovirus-based single-shot COVID-19 vaccine has EUA for adults aged 18 and older at least two months after the primary vaccination with its vaccine or with Pfizer or Moderna’s two-shot mRNA COVID-19 vaccine regimen. J&J’s booster shot is also authorized for use in Europe.
Pfizer and BioNTech both carry a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








