Pfizer
PFE
/
BioNTech
BNTX
announced that the conditional marketing authorization (CMA) for its two-shot vaccine for COVID-19, Comirnaty in the European Union (EU) has been expanded to allow vaccinating adolescents, 12 to 15 years of age. This is the first COVID-19 vaccine authorized in Europe for this age group.
The approval follows a positive opinion earlier granted by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) for extension of the CMA in the European Union. The CMA was originally issued on Dec 21, 2020 for vaccinating individuals 16 years of age and older.
Please note that in the United States, the vaccine was approved for this age group earlier this month. The U.S Centers for Disease Control and Prevention (CDC) also endorsed the safety and efficacy of Comirnaty/BNT162b2 for use in adolescents aged 12-15 years old. Vaccinations are in full swing in the country.
The approvals for the adolescent age group was based on data from a phase III study, which enrolled 2,260 adolescents. The study data showed that BNT162b2 was 100% effective in preventing COVID-19 in adolescents aged 12-15 years old. Moreover, in the pivotal phase III study, the candidate demonstrated robust antibody levels, exceeding those in participants aged 16-25 years old in another study. The vaccine was also well tolerated.
BNT162b2/Comirnaty was developed in record time and is now approved for emergency/temporary use in 91 countries worldwide. As of May 3, 2021, Pfizer/BioNTech shipped approximately 430 million doses of the vaccine to 91 countries.
Meanwhile studies are ongoing to expand the authorization of BNT162b2 to additional population groups, such as children from 6 months to 11 years of age and pregnant women. The companies are also evaluating a potential booster dose and an updated version of the vaccine.
Pfizer’s stock has risen 5.2% this year so far compared with an increase of 5.7% for the
industry
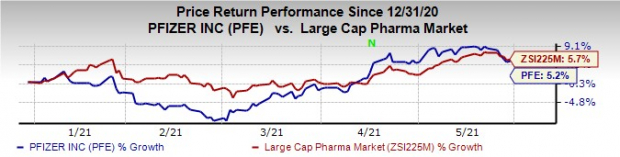
Image Source: Zacks Investment Research
BioNTech’s shares have surged 290% this year so far against the
industry
’s decrease of 8%.
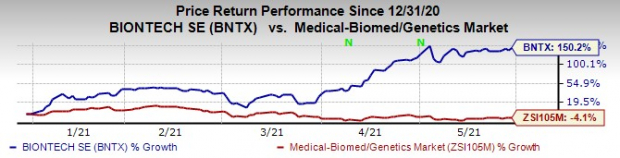
Image Source: Zacks Investment Research
Other marketed COVID-19 vaccines are
Moderna
’s
MRNA
mRNA-1273,
J&J
’s
JNJ
single-shot COVID-19 vaccine and
AstraZeneca
’s (AZN) COVID-19 vaccine. Moderna is currently evaluating its vaccine in a phase II/III study for adolescents. Last week, the
study met its primary endpoint
by demonstrating non-inferior immunogenicity compared to COVE study that evaluated the vaccine in adults.
Pfizer has a Zacks Rank #3 (Hold) while BioNTech sports a Zacks Rank #1 (Strong Buy). You can see
the complete list of today’s Zacks #1 Rank stocks here
.
Zacks’ Top Picks to Cash in on Artificial Intelligence
In 2021, this world-changing technology is projected to generate $327.5 billion in revenue. Now Shark Tank star and billionaire investor Mark Cuban says AI will create “”the world’s first trillionaires.”” Zacks’ urgent special report reveals 3 AI picks investors need to know about today.
See 3 Artificial Intelligence Stocks With Extreme Upside Potential>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








