Pfizer
PFE
and partner
BioNTech
BNTX
announced that they have entered into an agreement with the United States government to supply 105 million doses of their COVID-19 vaccine by this year. Upon receiving these doses, the U.S. government will pay $3.2 billion to the companies.
Per the terms of the agreement, Pfizer and BioNTech will supply three different doses of the vaccine, 30 µg, 10 µg and 3 µg. In fact, the agreement also includes the supply of Pfizer/BioNTech’s Omicron-adapted COVID-19 vaccineif they are granted emergency use authorization (EUA) by the FDA.
The agreement also provides the U.S. government with an option to purchase up to 195 million additional doses of Pfizer/BioNTech’s COVID-19 vaccine, bringing the total number of potential doses to 300 million.
Shares of Pfizer have lost 13.7% in the year so far against the
industry
’s 4.8% rise.
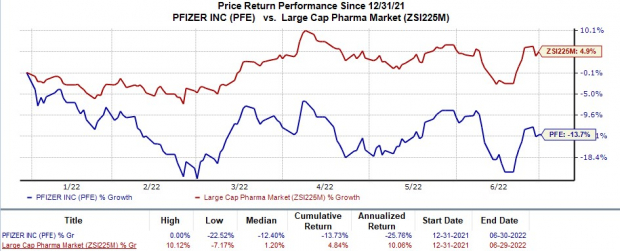
Image Source: Zacks Investment Research
The supply agreement with the U.S. government should help boost vaccines sales for full-year 2022 for both Pfizer and BioNTech, who jointly share the profits from the sale of the COVID-19 vaccine doses. In May, Pfizer said it expects to record $32 billion from the sales of COVID vaccines in 2022.
Last week, Pfizer and BioNTech announced new data from a phase II/III study, evaluating the safety, tolerability, and immunogenicity of two Omicron-adapted COVID-19 vaccine candidates – one monovalent and the other bivalent.
Data from the phase II/III study showed that participants given the booster dose of both the vaccine candidates generated a
substantially higher immune response
against the Omicron subvariant BA.1 when compared to Comirnaty, Pfizer/BioNTech’s authorized COVID-19 vaccine.
The bivalent vaccine is a combination of Comirnaty and a vaccine candidate targeting the spike protein of the Omicron BA.1 subvariant.
Pfizer has already shared data from studies on its two Omicron-adapted vaccine candidates with regulators, including the FDA, and a request for EUA is planned.
Pfizer and BioNTech have already started manufacturing doses of the two candidates in anticipation of an authorization for use from the FDA. Both companies intend to start delivery of the two vaccine candidates as soon as they gain FDA authorization.
The monovalent and bivalent vaccine candidates have been developed by Pfizer and BioNTech to address the rise of new and evolving Omicron subvariants. Not only do these variants outcompete the BA.1 subvariant but have also shown the potential to evade immune protection, which creates a need for adapted vaccine boosters.
The COVID-19 vaccines developed by Pfizer and BioNTech are based on mRNA technology. Another vaccine based on mRNA technology, which poses stiff competition to the Pfizer/BioNTech vaccine, has been developed by
Moderna
MRNA
.
Moderna is also working on developing a new Omicron-based COVID-19 vaccine. Earlier this month, Moderna announced data from an ongoing phase II/III study, which evaluated its bivalent COVID-19 vaccine booster candidate, mRNA-1273.214, targeting the Omicron variant.
The clinical data showed that following one month of administration of the 50 µg dose of mRNA-1273.214, a 5.4-fold
increase in neutralizing antibodies
against the Omicron subvariants, BA.4 and BA.5, was seen in all study participants, regardless of prior infection. In a subset of seronegative participants, the candidate generated a 6.3-fold increase in neutralizing titers against both the Omicron subvariants. Based on these results, Moderna intends to complete the regulatory filing for mRNA-1273.214 in the coming weeks.
This week, the FDA’s Vaccines and Related Biological Products Advisory Committee (“VRBPAC”) voted 19-2 to recommend
modifying the current strain composition
of available COVID-19 vaccines to target the Omicron variant and its subvariants. If the FDA follows the VRBPAC’s recommendation, which it usually does, Pfizer/BioNTech will need to roll out Omicron-based vaccine boosters to provide better protection from the virus, which has been mutating rapidly.
The Biden government expects to start a booster campaign in October to prepare for an expected surge in infections in autumn. Pfizer’s latest deal with the U.S. government looks like a step in this direction.
Zacks Rank & Stocks to Consider
Pfizer currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is
Sesen Bio
SESN
, which sports a Zacks Rank #1 (Strong Buy) at present. You can see
the complete list of today’s Zacks #1 Rank stocks here
.
Estimates for Sesen Bio’s 2022 bottom line have declined from a loss of 46 cents to 44 cents in the past 60 days. Share prices of Sesen Bio have fallen 2.2% in the year-to-date period.
Earnings of Sesen Bio beat estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 69.9%. In the last reported quarter, Sesen Bio delivered an earnings surprise of 100%.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









