Pfizer
PFE
announced that the FDA has accepted the biologics license application (BLA) seeking approval of its pentavalent meningococcal vaccine candidate (MenABCWY).
The BLA seeks approval of MenABCWY for adolescents and young adults, 10-25 years of age, for the prevention of meningococcal disease caused by five meningococcal serogroups that cause the majority of invasive meningococcal disease: serogroups A, B, C, W and Y.
The FDA has assigned a standard review to the BLA, with a decision expected in October 2023.
Pfizer’s stock is down 14% this year so far against an increase of 12.1% for the
industry
.
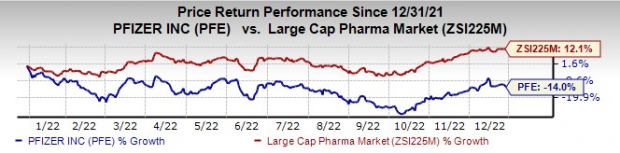
Image Source: Zacks Investment Research
The BLA application as based on data from a phase III study, which evaluated MenABCWY in healthy individuals 10 through 25 years of age. The study met all primary and secondary endpoints. In the study, the vaccine candidate was well tolerated with an acceptable safety profile. The protection provided by two doses of MenABCWY was non-inferior to licensed vaccines (two doses of Pfizer’s own Trumenba + one dose of
GSK
’s [
GSK
] Menveo) for serogroups A, B, C, W and Y.
The 5-in-1 meningitis vaccine candidate has the potential to simplify the meningococcal vaccination schedule by reducing the number of injections to be fully vaccinated compared to current FDA-approved meningitis vaccines as it has been designed to protect against the five serogroups with one combined product.
GSK is also developing two MenABCWY pentavalent (5-in-1) vaccines. The first generation is in late-stage development and the second generation is in an earlier stage.
MenABCWY is one of the five new molecular entities that Pfizer expects to launch in 2023. The other products include a respiratory syncytial virus (RSV) vaccine, ritlecitinib for alopecia, elranatamab for multiple myeloma and Abrilada, a biosimilar of
AbbVie
’s
ABBV
Humira.
A BLA seeking approval of Pfizer’s RSV vaccine is under FDA’s priority review, with a decision expected in May 2023. GSK’s RSV vaccine for older adults is also under FDA’s priority review, with a decision expected on May 3, 2023. Regulatory applications seeking approval of GSK’s RSV vaccine are under review in Japan and Europe as well.
Ritlecitinib is also under review in Europe and the United States, with an FDA decision expected in second-quarter 2023. The FDA approved Abrilada, Pfizer’s biosimilar of AbbVie’s Humira in November 2019 but the product will be launched in 2023 once AbbVie loses exclusivity for the branded product. Abrilada is the fifth FDA-approved biosimilar to Humira.
Zacks Rank & Stocks to Consider
Pfizer currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
A better-ranked large drugmaker is
Sanofi
SNY
, which carries a Zacks Rank #2 (Buy)
Sanofi stock has declined 4.6% this year so far. Estimates for Sanofi’s 2022 earnings have gone up from $4.04 to $4.27 per share, while that for 2023 have increased from $4.22 to $4.31 per share over the past 60 days.
Sanofi beat earnings expectations in all the trailing four quarters. The company delivered a four-quarter earnings surprise of 9.50%, on average.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








