Pfizer Inc.
PFE
announced that the FDA has granted Breakthrough Therapy designation to its respiratory syncytial virus (“RSV”) vaccine candidate, PF-06928316 or RSVpreF, to prevent lower respiratory tract disease caused by RSV in individuals aged 60 years and above.
A Breakthrough Therapy status is granted to medicines being evaluated for serious conditions where early clinical evidence indicates said medicines’ effectiveness for substantial improvement over available therapies.
Per the press release, the above designation was based on positive data from a proof-of-concept phase IIb study evaluating the safety, immunogenicity and efficacy of a single dose of RSVpreF (120µg) in a human viral challenge model in healthy adults aged between 18 to 50 years.
Shares of Pfizer have dipped 10.9% in the year so far against the
industry
’s rise of 1.5%.
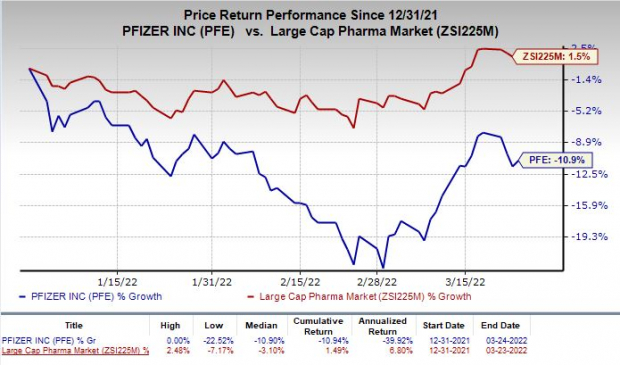
Image Source: Zacks Investment Research
The ongoing phase III RENOIR study,
initiated
in September 2021, is currently evaluating the safety, immunogenicity and efficacy of a single dose of RSVpreF in adults aged 60 years or older.
Earlier this month, RSVpreF was granted Breakthrough Therapy designation by the FDA for active immunization of pregnant women to prevent RSV-associated lower respiratory tract illness in infants from birth up to six months of age.
Pfizer is currently evaluating RSVpreF in a phase III study for vaccination against RSV infection in infants.
Per the company, RSVpreF has the potential to become the first RSV vaccine that will help protect infants in their vulnerable first months of life from the disease caused by the highly-contagious RSV virus.
RSV is a pervasive cause of acute respiratory illness that usually starting in the fall months. The highly contagious virus affects the lungs and airways. Currently, there are no FDA-approved RSV vaccines, implying an attractive opportunity in said field.
Some other companies are also developing their respective vaccines for treating RSV.
AstraZeneca
AZN
and
Sanofi
SNY
are evaluating a single dose of their RSV vaccine candidate, nirsevimab, for the protection of infants.
Earlier this month, AZN and SNY published detailed data from their joint late-stage study, MELODY. A single dose of AstraZeneca and Sanofi’s nirsevimab reduced LRTI like bronchiolitis or pneumonia caused by RSV by 74.5% compared to placebo in infants born at term or late preterm.
AstraZeneca and Sanofi are conducting another phase II/III study, MEDLEY, evaluating nirsevimab in preterm infants and infants with congenital heart disease and chronic lung disease entering the first RSV season.
Glaxo
GSK
is evaluating its maternal RSV vaccine candidate in the phase III GRACE study. The study is evaluating the efficacy of a single dose of the unadjuvanted candidate in pregnant women.
Glaxo is also developing RSV vaccines for patients aged 60 years and above in a phase III study. GSK expects data readout from this study in the first half of 2022.
Zacks Rank
Pfizer currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +25.4% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








