This week, the FDA approved
Roche
’s
RHHBY
Lunsumio, a first-in-class bispecific antibody, for relapsed or refractory follicular lymphoma (FL), the most common slow-growing form of non-Hodgkin lymphoma. The FDA also accepted
Pfizer
’s
PFE
biologics license application (BLA) for a pentavalent meningococcal vaccine candidate (MenABCWY). The Japanese regulatory authority approved a combination of
AstraZeneca
’s
AZN
immunotherapies, Imfinzi (durvalumab) and Imjudo (tremelimumab), for two cancer types, advanced liver and lung cancer.
Recap of the Week’s Most Important Stories
FDA Approves Roche’s Lunsumio (mosunetuzumab):
The FDA approved Roche’s CD20xCD3 T cell engaging bispecific antibody,
Lunsumio (mosunetuzumab) , for treating relapsed or refractory follicular lymphoma
. The accelerated approval is for adult patients who have been treated with two or more lines of systemic therapy. Lunsumio was approved for a similar indication in the European Union earlier this June. Off-the-shelf cancer immunotherapy, Lunsumio will help treat patients who do not respond to multiple lines of treatment and help them achieve disease remission. A commercial launch of the therapy is expected in the coming weeks.
FDA’s Accepts Pfizer’s Meningococcal Vaccine BLA:
The
FDA accepted and granted standard review
to Pfizer’s biologics license application (BLA) seeking approval of its pentavalent meningococcal vaccine candidate (MenABCWY). The BLA seeks approval of MenABCWY for adolescents and young adults, 10-25 years of age, for the prevention of meningococcal disease caused by five meningococcal serogroups that cause the majority of invasive meningococcal disease: serogroups A, B, C, W and Y. The FDA assigned a standard review to the BLA, with a decision expected in October 2023. A pentavalent vaccine, if approved, can simplify what is currently a complex meningococcal vaccination schedule in the country.
Pfizer’s phase III study on hemophilia B gene therapy candidate, fidanacogene elaparvovec, met its primary endpoint. The primary endpoint of the study was non-inferiority and superiority in the annualized bleeding rate (ABR) of total bleeds post-fidanacogene elaparvovec infusion versus prophylaxis regimen with Factor IX (FIX), administered as part of regular care. In the study, a single dose of 5e11 vg/kg of fidanacogene elaparvovec resulted in a 71% reduction in ABR. Key secondary endpoints demonstrated a 78% reduction in treated ABR and a 92% reduction in annualized infusion rate. Pfizer plans to share this data with regulatory authorities in early 2023.
AstraZeneca’s Imfinzi+Imjudo Gets Japan Nod for 3 Cancers:
The Japanese Ministry of Health, Labour and Welfare (MHLW) approved AstraZeneca’s dual immunotherapy regimen, comprising PD-L1 inhibitor Imfinzi plus CTLA-4 antibody Imjudo (tremelimumab), in combination with chemotherapy, for treating unresectable, advanced or recurrent non-small cell lung cancer (NSCLC). The MHLW also approved the Imfinzi+Imjudo combination for patients with unresectable hepatocellular carcinoma (HCC), the most common type of liver cancer. Imfinzi plus Imjudo was approved by the FDA for advanced liver and NSCLC cancers in October/November. The Japanese regulatory authorities also approved Imfinzi as monotherapy for unresectable HCC and curatively unresectable biliary tract cancer in combination with chemotherapy.
The MHLW also granted approval to AstraZeneca’s BTK inhibitor, Calquence as a first-line treatment for adult patients with chronic lymphocytic leukemia (CLL) (including small lymphocytic lymphoma). Calquence is already approved in Japan for treating relapsed or refractory CLL. It is approved for both treatment-naïve and relapsed or refractory settings in several countries, including the United States and Europe, and also for mantle cell lymphoma in the United States and some other countries.
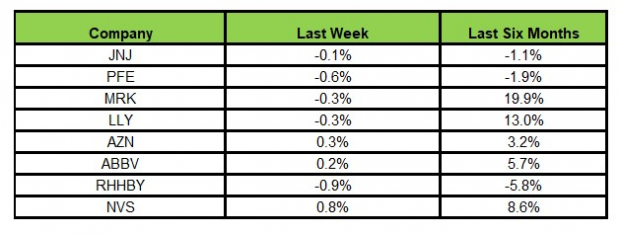
The NYSE ARCA Pharmaceutical Index rose 0.25% in the last five trading sessions.
Zacks Top 10 Stocks for 2023
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2023? From inception in 2012 through November, the
Zacks Top 10 Stocks
portfolio has tripled the market, gaining an impressive +884.5% versus the S&P 500’s +287.4%.
Now our Director of Research is combing through 4,000 companies covered by the Zacks Rank to handpick the best 10 tickers to buy and hold. Don’t miss your chance to get in on these stocks when they’re released on January 3.
Be First to New Top 10 Stocks >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report