This week, the FDA granted emergency approval to
Pfizer
PFE
and BioNTechand
Moderna’
s
MRNA
vaccines for young children. The FDA also approved the expanded use of
AbbVie
’s
ABBV
immunology drug, Skyrizi,
Merck
’s
MRK
pneumococcal conjugate vaccine, Vaxneuvance and
Novartis
’
NVS
cancer combination drugs, Tafinlar (dabrafenib) + Mekinist (trametinib).
Recap of the Week’s Most Important Stories
FDA Authorizes Covid Vaccines of Pfizer, Moderna for Kids Under 5:
The FDA granted emergency approval to Pfizer/BioNTech and Moderna’s mRNA-based COVID-19 vaccines for young children. While Pfizer and BioNTech get emergency use authorization (EUA) for their
COVID-19 vaccine for children six months to less than five years
of age as a three-dose vaccine, Moderna received EUA for two 25-µg doses of its vaccine in children six months to under six years of age. Pfizer’s formulation of a booster dose for these youngest children is 3-µg, which is one-tenth of the dose strength for adults.
Kids under five years of age were, until now, not authorized to get any COVID-19 vaccine in the United States. Moderna also received EUA for a 50 μg two-dose regimen of its vaccine for children aged 6 through 11 years old and a 100 μg two-dose regimen for adolescents aged 12 through 17 years old.
The U.S. Centers for Disease Control and Prevention (CDC) also recommended a COVID vaccine for all children 6 months through 5 years of age.
FDA Approves AbbVie Skyrizi for Crohn’s Disease:
The FDA approved AbbVie’s Skyrizi (risankizumab) for its
third indication
, moderately to severely active Crohn’s disease (“CD”). In September last year, the FDA delayed its decision on Skyrizi for the CD indication by three months as it needed time to review the additional information filed by AbbVie, including information about the on-body injector. Skyrizi’s approval for the CD indication was supported by data from two phase III induction studies — ADVANCE, MOTIVATE, and one maintenance study — FORTIFY. In the studies, Skyrizi demonstrated significant improvements in both the co-primary endpoints of endoscopic response and clinical remission as an induction and maintenance therapy.
Skyrizi is presently approved to treat moderate-to-severe plaque psoriasis and active psoriatic arthritis. Skyrizi is under review in Europe for the CD indication.
AbbVie submitted a supplemental new drug application (sNDA) with the FDA seeking approval for its CGRP receptor antagonist, Qulipta (atogepant), for the prevention of chronic migraine. Qulipta was approved for the preventive treatment of episodic migraine in adults in September last year. With the latest approval for chronic migraine prevention, Qulipta becomes the only CGRP receptor antagonist approved for the
broad preventive treatment of migraine indication
, including episodic and chronic.
FDA Approves Merck’s Vaxneuvance Vaccine for Kids:
The FDA approved Merck’s pneumococcal 15-valent conjugate vaccine, Vaxneuvance,
for use in children 6 weeks through 17 years of age
to protect against invasive pneumococcal disease. The vaccine was approved in the United States in July and in Europe in December last year for use in adults. Merck’s Vaxneuvance vaccine includes pneumococcal serotypes 22F and 33F, which are not included in the currently licensed 13-valent pneumococcal conjugate vaccines (PCV13).
The CDC’s Advisory Committee on Immunization Practices (ACIP) also unanimously voted to provisionally recommend Vaxneuvance as an option for vaccination in infants and children, including routine use in children under 2 years of age.
Pfizer to Buy 8.1% Stake in Partner Valneva:
Pfizer acquired an 8.1% stake in its French partner Valneva SE
with whom it is co-developing VLA15
, a vaccine candidate for Lyme disease. Valneva will use the cash from this investment to support its phase III study for VLA15. A phase III study of the vaccine is expected to start in third-quarter 2022. The companies also updated the terms of their collaboration and license agreement, which they had formed in 2020. Valneva will now fund 40% of the cost of developing the vaccine, up from 30% initially agreed upon.
Novartis’ Approvals for Cancer Drugs:
The FDA granted accelerated approval to Novartis’ Tafinlar (dabrafenib) + Mekinist (trametinib) combination for a tumor-agnostic indication for adult and pediatric patients with solid tumors that have a BRAF V600E mutation, the most common type of BRAF mutation. The approval was based on positive data from the phase II ROAR and NCI-MATCH studies. The studies showed overall response rates up to 80% in patients with BRAF V600E solid tumors treated with the Tafinlar + Mekinist combination.
The European Commission approved Novartis’ Tabrecta (capmatinib) as monotherapy for previously-treated patients with METex14 skipping advanced non-small cell lung cancer. The approval was based on the phase II GEOMETRY mono-1 study. Tabrecta is already approved in the United States for a similar indication.
The NYSE ARCA Pharmaceutical Index rose 5.41% in the last five trading sessions.
Here’s how the eight major stocks performed in the last five trading sessions.
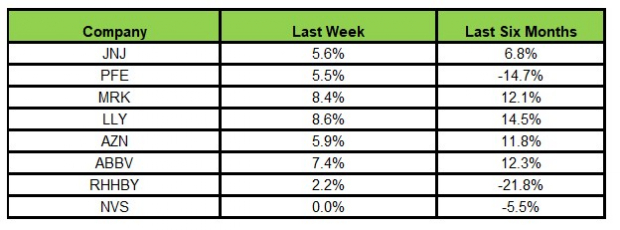
Image Source: Zacks Investment Research
All the stocks were in the green in the last five trading sessions. Lilly rose the most (8.6%).
In the past six months, Lilly rose the most (14.5%) while Roche declined the most (21.8%).
(See the last pharma stock roundup here:
FDA Panel Nod for PFE, MRNA Young Kids’ COVID Jabs & More
)
What’s Next in the Pharma World?
Watch for regular pipeline and regulatory updates next week.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +25.4% per year. So be sure to give these hand-picked 7 your immediate attention.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








