Prothena Corporation
PRTA
announced a collaboration with Denmark-based
Novo Nordisk
NVO
, whereby the latter will acquire Prothena’s clinical-stage antibody, PRX004, and broader ATTR amyloidosis program.
In exchange, Prothena is eligible to receive development and sales milestone payments of approximately $1.2 billion, including $100 million in upfront and near-term clinical milestone payments.
The candidate is a phase II-ready, anti-amyloid immunotherapy designed to deplete the amyloid deposits that are associated with the disease pathology of ATTR amyloidosis. Prothena has completed a phase I open-label, multicenter dose-escalation study of PRX004 in patients with hereditary forms of ATTR. In the study, 21 patients with hereditary ATTR Amyloidosis (hATTR) were enrolled to receive PRX004 intravenously once every 28 days for up to 3 infusions in the dose-escalation phase of the study. The eligible patients who completed dose-escalation were provided the opportunity to enroll in the long-term extension (LTE) portion of the study.
Novo Nordisk will develop the phase II antibody, PRX004, for the rare heart disease, ATTR cardiomyopathy, which is an underdiagnosed and potentially fatal form of ATTR amyloidosis, characterized by the build-up of amyloid deposits in cardiac tissue.
The decision to sell its ATTR amyloidosis program comes at a strategic point for the company. The company’s pipeline includes programs for the potential treatment of Alzheimer’s disease (AD), including PRX012 that targets Aβ (Amyloid beta). The partnered programs include prasinezumab, in collaboration with
Roche
RHHBY
, for the potential treatment of Parkinson’s disease and other related synucleinopathies, and programs that target tau (PRX005), TDP-43, and an undisclosed target in collaboration with Bristol Myers Squibb for the potential treatment of AD, amyotrophic lateral sclerosis (ALS), or other neurodegenerative diseases.
Shares of the company have surged 352.9% in the year so far against the
industry
‘s decline of 2%.
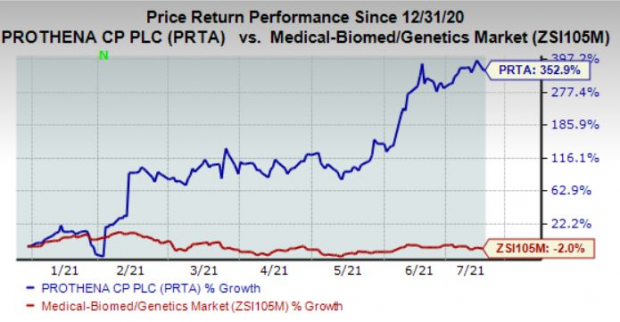
Image Source: Zacks Investment Research
Most of the upside came after the FDA approval of
Biogen
’s
BIIB
Alzheimer’s disease (AD) drug, Aduhelm. On Jun 7, 2021, Biogen and partner Eisai won the FDA approval for Aduhelm (aducanumab-avwa) as the first and only AD treatment.
The approval has brought the spotlight on other companies as well who are developing drugs for AD and investors are optimistic about the prospects of these pipeline candidates. Prothena’s PRX005 is being evaluated for the treatment of AD. The company has another candidate, PRX012, which is a potential treatment for AD.
The divestment of its ATTR amyloidosis program will enable Prothena to focus on its neurodegenerative drugs.
Meanwhile, the abovementioned acquisition will diversify Novo Nordisk’s diabetes and obesity portfolio.
Prothena currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Bitcoin, Like the Internet Itself, Could Change Everything
Blockchain and cryptocurrency has sparked one of the most exciting discussion topics of a generation. Some call it the “Internet of Money” and predict it could change the way money works forever. If true, it could do to banks what Netflix did to Blockbuster and Amazon did to Sears. Experts agree we’re still in the early stages of this technology, and as it grows, it will create several investing opportunities.
Zacks’ has just revealed 3 companies that can help investors capitalize on the explosive profit potential of Bitcoin and the other cryptocurrencies with significantly less volatility than buying them directly.
See 3 crypto-related stocks now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








