Quest Diagnostics Incorporated
DGX
has been granted a contract by the Centers for Disease Control and Prevention (CDC) to conduct testing and provide laboratory data analysis to help detect patterns in SARS-COV-2 seroprevalence on a multi-state basis. The contract was awarded following a competitive bid, with the overall contract value at $19.5 million, including all options.
The contract is intended to assist the CDC in evaluating the proportion of the population infected by or vaccinated against SARS-CoV-2. It expands Quest Diagnostics’ contributions to the CDC’s seroprevalence research, including participation in the SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology and Surveillance consortium.
Per management, the company offers a powerful mix of quality COVID-19 serological testing, nationwide reach and robust data analytics to guide public health strategies. The assets will contribute to the CDC’s efforts to track the country’s COVID-19 vulnerability.
Efforts to Assess COVID-19 Vulnerability
With its comprehensive list of COVID-19 serological tests, Quest Diagnostics will leverage serum specimen remnants from clinical testing for a range of non-COVID-19 conditions to detect the immune response to SARS-CoV-2.
Notably, the company has gained the FDA approval for its serological tests for emergency use and for helping identify antibodies produced in response to recent or prior infection and/or vaccination.
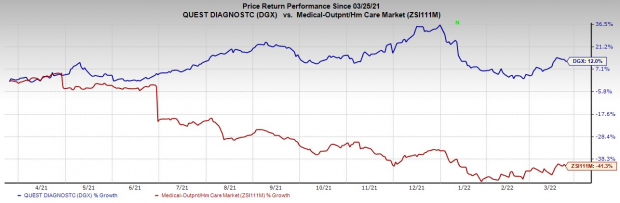
Image Source: Zacks Investment Research
The company will further deliver the data analysis to CDC in a HIPAA-compliant manner to support public health analysis and reporting.
More on the News
Quest Diagnostics partnered with the CDC on various initiatives to improve population health analysis based on insights from its national testing data. The company signed an agreement in January 2021 to deliver SARS-CoV-2 variant sequencing to aid in the identification and tracking of new variants.
The company also collaborated with the CDC on research to determine the impacts of the pandemic on non-prescribed fentanyl usage in individuals with opioid use disorder, as well as hepatitis C screening and diagnostic patterns.
Industry Prospects
Per a report
published in Grand View Research, the global COVID-19 diagnostics market size is expected to see a CAGR of 3.1% between 2021 and 2027. Factors, including increasing government initiatives to implement mass testing, the growing demand for COVID-19 diagnostic products, new entrants shifting their focus to at-home testing and several testing programs to help the travel industry gear up, are fuelling market growth.
Given the market prospects, Quest Diagnostics’ latest CDC contract seems strategic.
Other Notable Developments
The company engaged in a number of significant developments in January 2022.
Quest Diagnostics announced the availability of an at-home COVID-19 rapid antigen test service for consumer purchase through its consumer-initiated testing platform, QuestDirect.
The new service features proctored telehealth visits in partnership with eMed to help individuals meet travel, other observed collection and test report criteria. The service allows individuals to use Abbott BinaxNOW COVID-19 Ag Card Home Test under the supervision of a certified telehealth proctor. The laboratory report for the same can be obtained through eMed.
Quest Diagnostics introduced the Comprehensive Health Profile from QuestDirect. The innovative offering assesses more than 100 health-related data points to provide individuals with a personalized picture of their overall health. Following nearly two years of disrupted or delayed healthcare due to the pandemic, the latest offering will allow individuals to access healthcare at their convenience.
Share Price Performance
The DGX stock has outperformed its
industry
over the past year. It has gained 12% against the industry’s 41.3% decline.
Zacks Rank and Key Picks
Currently, Quest Diagnostics carries a Zacks Rank #3 (Hold).
A few better-ranked stocks in the broader medical space are
Henry Schein, Inc.
HSIC
,
McKesson Corporation
MCK
and
IDEXX Laboratories, Inc.
IDXX
.
Henry Schein has an estimated long-term growth rate of 11.8%. HSIC’s earnings surpassed estimates in the trailing four quarters, the average surprise being 25.5%. It currently has a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Henry Schein has outperformed the industry over the past year. HSIC has gained 31.4% compared with the industry’s 8% rise over the past year.
McKesson has a long-term earnings growth rate of 11.8%. MCK’s earnings surpassed estimates in the trailing four quarters, delivering a surprise of 20.6%, on average. It presently carries a Zacks Rank #2.
McKesson has outperformed the industry over the past year. MCK has gained 56.7% in the said period compared with 8% growth of the industry.
IDEXX has a long-term earnings growth rate of 13%. The company’s earnings surpassed estimates in the trailing four quarters, delivering an average surprise of 18.6%. IDXX currently has a Zacks Rank #2.
IDEXX has outperformed its industry in the past year. IDXX has gained 13.5% compared with the industry’s 1.8% fall in the same period.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top buy-and-hold tickers for the entirety of 2022?
Last year’s 2021
Zacks Top 10 Stocks
portfolio returned gains as high as +147.7%. Now a brand-new portfolio has been handpicked from over 4,000 companies covered by the Zacks Rank. Don’t miss your chance to get in on these long-term buys
Access Zacks Top 10 Stocks for 2022 today >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








